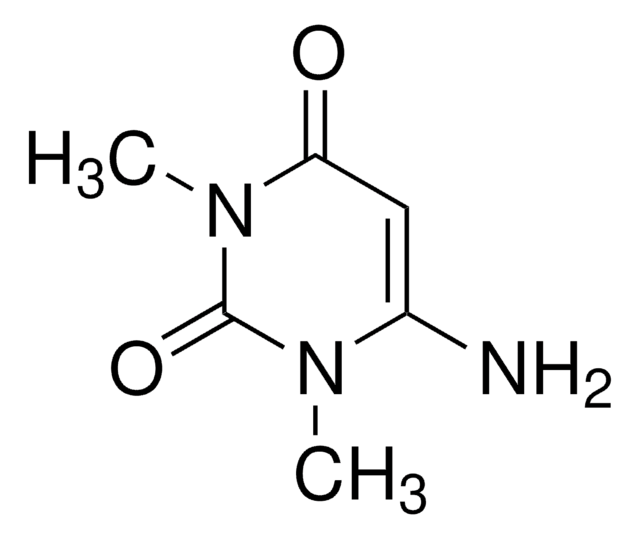
おすすめの製品
アッセイ
99%
形状
powder
mp
119-122 °C (lit.)
SMILES記法
CN1C=CC(=O)N(C)C1=O
InChI
1S/C6H8N2O2/c1-7-4-3-5(9)8(2)6(7)10/h3-4H,1-2H3
InChI Key
JSDBKAHWADVXFU-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
1,3-Dimethyluracil is a pyrimidine derivative. Stability of the C6-centered carbanions derived from 1,3-dimethyluracil has been investigated in the gas phase and in DMSO and water solutions. The excited state structural dynamics of 1,3-dimethyluracil (DMU) in water and acetonitrile has been studied by resonance Raman spectroscopy. Crystal structure of 1,3-dimethyluracil has been reported. Ultraviolet irradiation of aqueous 1,3-dimethyluracil results in hydration of the 5:6 double bond of the uracil ring to form 1,3-dimethyl-6-oxy-hydrouracil.
アプリケーション
1,3-Dimethyluracil is suitable reagent used to investigate the steady-state absorption and fluorescence spectra of uracil derivatives. It may be used in the preparation of 2,6-dihydroxynicotinamide.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
349801-BULK:
349801-1G:
349801-250G:
349801-5G:
349801-VAR:
Adam Gryff-Keller et al.
The journal of physical chemistry. A, 116(39), 9632-9638 (2012-09-14)
The practical utility of the method of retrieving the relaxation rate of a quadrupole nucleus via the scalar relaxation of the second kind (SC2) of an I = 1/2 spin nucleus has been considered once again. The study was motivated
Nicholas A Senger et al.
Tetrahedron, 69(26), 5287-5292 (2013-09-28)
The stabilities of the C6-centered carbanions derived from 1,3-dimethyluracil, N-methyl-2-pyridone, and N-methyl-4-pyridone were systematically investigated in the gas phase and in DMSO and water solutions. The stabilities of the carbanions in the gas phase and DMSO were directly measured through
Probing noncovalent interactions in biomolecular crystals with terahertz spectroscopy.
Thomas Kleine-Ostmann et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 9(4), 544-547 (2008-02-15)
H P Schuchmann et al.
International journal of radiation biology and related studies in physics, chemistry, and medicine, 50(6), 1051-1068 (1986-12-01)
Hydroxymethyl radicals .CH2OH, generated by the radiolysis of methanol (0.5 mol dm-3) in N2O-saturated aqueous solutions, were reacted with 1,3-dimethyluracil or 1,3-dimethylthymine (10(-3) mol dm-3). The products were identified and their G values determined. It has been concluded that in
Anna A Zadorozhnaya et al.
The journal of physical chemistry. A, 114(4), 2001-2009 (2010-01-09)
The electronic structure of 1,3-dimethyluracil and its dimer is characterized by ab initio calculations. The methylation eliminates the H-bonded isomers and allows one to focus on the pi-stacked manifold. In the neutral species, methylation increases the binding energy by 3-4
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)