おすすめの製品
製品名
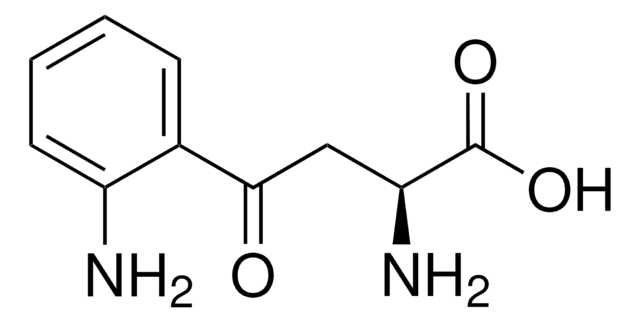
L-キヌレニン 硫酸塩, crystalline
アッセイ
≥98% (HPLC)
品質水準
フォーム
crystalline
テクニック
ligand binding assay: suitable
色
light yellow
保管温度
−20°C
SMILES記法
OS(O)(=O)=O.N[C@@H](CC(=O)c1ccccc1N)C(O)=O
InChI
1S/C10H12N2O3.H2O4S/c11-7-4-2-1-3-6(7)9(13)5-8(12)10(14)15;1-5(2,3)4/h1-4,8H,5,11-12H2,(H,14,15);(H2,1,2,3,4)/t8-;/m0./s1
InChI Key
KAXRWMOLNJZCEW-QRPNPIFTSA-N
アプリケーション
L-Kynurenine sulfate salt has been used as a substrate to study the enzyme activity of kynurenine aminotransferase. It has also been used in the synthesis of Kyn adducts of certain amino acids .
生物化学的/生理学的作用
L-Kynurenine (L-Kyn) is a precursor of kynurenic acid which is the only recognized endogenous excitatory amino acid receptor antagonist in the central nervous system. L-Kyn is known to be a pigment generating component in animals. In mammals, it modulates the transmission of glutamate neurotransmitter. It is also considered to be a sex-attracting pheromone in vertebrates.
L-Kynurenine is a key intermediate in the breakdown of L-tryptophan and the formation of nicotinamide adenine dinucleotide (NAD+) via the kynurenine pathway. L-kynurenine is involved in a variety of neurological processes and diseases. L-kynurenine is a substrate for kynureninase/kynurenine hydrolase; kynurenine 3-monooxygenase and kynurenine-oxoglutarate transaminase.
トリプトファンの分解経路における重要な中間体です。
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
K3750-VAR:
K3750-100MG:
K3750-1G:
K3750-500MG:
K3750-5G:
K3750-BULK:
この製品を見ている人はこちらもチェック
Novel protein modification by kynurenine in human lenses.
Vazquez S
The Journal of Biological Chemistry, 277(7), 4867-4873 (2002)
Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain.
Guidetti P
Journal of Neurochemistry, 102(1), 103-111 (2007)
K Nagy et al.
Bioorganic & medicinal chemistry, 19(24), 7590-7596 (2011-11-15)
The overactivation of excitatory amino acid receptors plays a key role in the pathomechanism of several neurodegenerative disorders and in ischemic and post-ischemic events. Kynurenic acid (KYNA) is an endogenous product of the tryptophan metabolism and, as a broad-spectrum antagonist
L-Kynurenine, an amino acid identified as a sex pheromone in the urine of ovulated female masu salmon.
Yambe H
Proceedings of the National Academy of Sciences of the USA, 103(42), 15370-15374 (2006)
Neuroprotective effects of L-kynurenine on hypoxia-ischemia and NMDA lesions in neonatal rats.
Nozaki K and Beal MF
Journal of Cerebral Blood Flow and Metabolism, 12(3), 400-407 (1992)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)