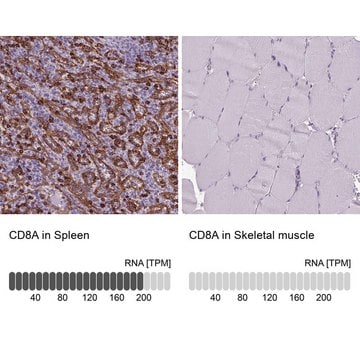
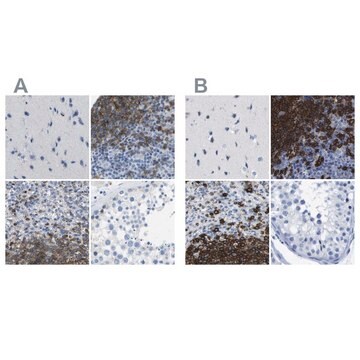
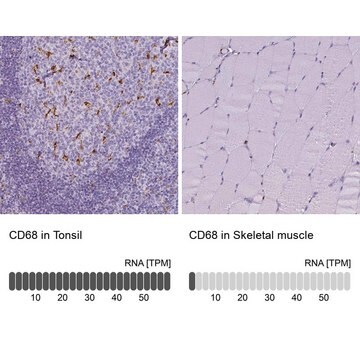
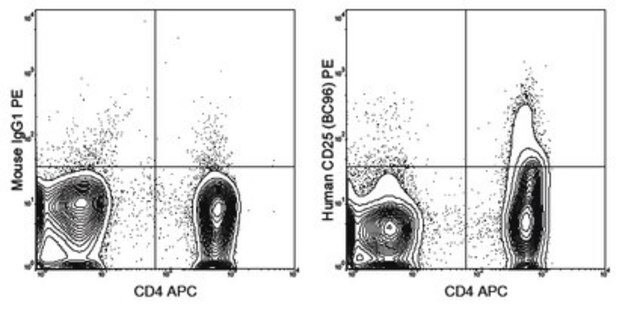
HPA014341
Anti-MS4A1 antibody produced in rabbit

Prestige Antibodies® Powered by Atlas Antibodies, affinity isolated antibody, buffered aqueous glycerol solution
別名:
Anti-B-lymphocyte antigen CD20, Anti-B-lymphocyte surface antigen B1, Anti-Bp35, Anti-Leu-16, Anti-Membrane-spanning 4-domains subfamily A member 1
About This Item
おすすめの製品
由来生物
rabbit
品質水準
結合体
unconjugated
抗体製品の状態
affinity isolated antibody
抗体製品タイプ
primary antibodies
クローン
polyclonal
製品種目
Prestige Antibodies® Powered by Atlas Antibodies
フォーム
buffered aqueous glycerol solution
交差性
human
強化検証
independent
orthogonal RNAseq
Learn more about Antibody Enhanced Validation
テクニック
immunoblotting: 0.04-0.4 μg/mL
immunofluorescence: 0.25-2 μg/mL
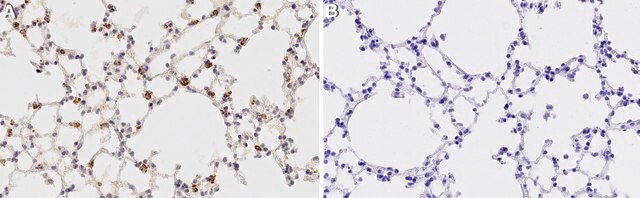
immunohistochemistry: 1:1000-1:2500
免疫原配列
ENEWKRTCSRPKSNIVLLSAEEKKEQTIEIKEEVVGLTETSSQPKNEEDIEIIPIQEEEEEETETNFPEPPQDQESSP
UniProtアクセッション番号
輸送温度
wet ice
保管温度
−20°C
ターゲットの翻訳後修飾
unmodified
遺伝子情報
human ... MS4A1(931)
詳細
免疫原
アプリケーション
生物化学的/生理学的作用
特徴および利点
Every Prestige Antibody is tested in the following ways:
- IHC tissue array of 44 normal human tissues and 20 of the most common cancer type tissues.
- Protein array of 364 human recombinant protein fragments.
関連事項
物理的形状
法的情報
免責事項
適切な製品が見つかりませんか。
製品選択ツール.をお試しください
保管分類コード
10 - Combustible liquids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
HPA014341-25UL:
HPA014341-100UL:
最新バージョンのいずれかを選択してください:
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)