おすすめの製品
製品種目
Duolink®
品質水準
テクニック
proximity ligation assay: suitable
蛍光検出
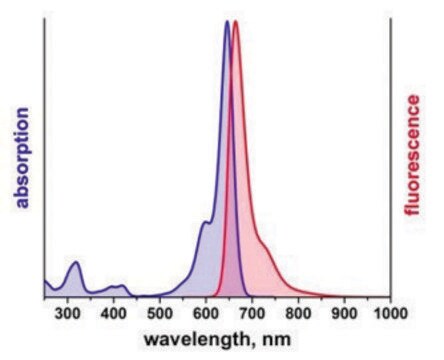
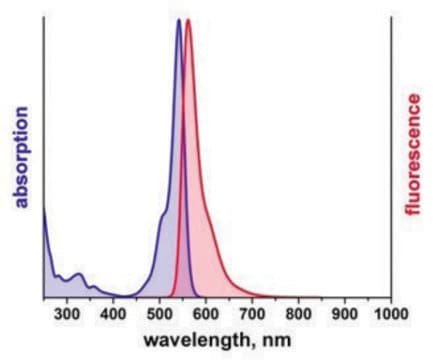
λex 360 nm; λem 460 nm
適合性
suitable for fluorescence-detection automated sequencing
suitable for microtiter plates
輸送温度
dry ice
保管温度
−20°C
アプリケーション
Use the Multiwell Plates modifications to the Duolink® In Situ Fluorescence Protocol to run an experiment with this product. A set of short instructions can also be used.
Visit our Duolink® PLA Resource Center for information on how to run a Duolink® experiment, applications, troubleshooting, and more.
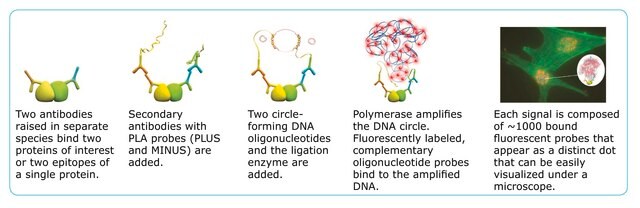
To perform a complete Duolink® PLA in situ experiment you will need two primary antibodies (PLA, IHC, ICC or IF validated) that recognize two target epitopes. Other necessary reagents include a pair of PLA probes from different species (one PLUS and one MINUS), detection reagents, wash buffers, and mounting medium. Note that the primary antibodies must come from the same species as the Duolink® PLA probes. Analysis is carried out using standard immunofluorescence assay equipment.
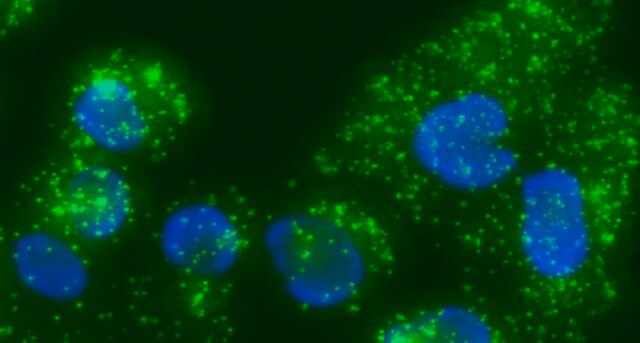
Duolink® In Situ Microplate Nuclear Stain and Anti-Fade are intended to be used after staining cells with Duolink® In Situ in microtiter plates. See the datasheet for more information.
Application Note
Two primary antibodies raised in different species are needed. Test your primary antibodies (IgG-class, mono- or polyclonal) in a standard immunofluorescence (IF), immunohistochemistry (IHC) or immunocytochemistry (ICC) assay to determine the optimal fixation, blocking, and titer conditions. Duolink® in situ reagents are suitable for use on fixed cells, cytospin cells, cells grown on slide, formalin-fixed, paraffin embedded (FFPE), or tissue (fresh or frozen). No minimum number of cells is required.
Let us do the work for you, learn more about our Custom Service Program to accelerate your Duolink® projects
View full Duolink® product list
特徴および利点
- No overexpression or genetic manipulation required
- High specificity (fewer false positives)
- Single molecule sensitivity due to rolling circle amplification
- Relative quantification possible
- No special equipment needed
- Quicker and simpler than FRET
- Increased accuracy compared to co-IP
- Publication-ready results
法的情報
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Aquatic Chronic 3 - Skin Sens. 1
保管分類コード
12 - Non Combustible Liquids
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
毒物及び劇物取締法
キットコンポーネントの情報を参照してください
PRTR
キットコンポーネントの情報を参照してください
消防法
キットコンポーネントの情報を参照してください
労働安全衛生法名称等を表示すべき危険物及び有害物
キットコンポーネントの情報を参照してください
労働安全衛生法名称等を通知すべき危険物及び有害物
キットコンポーネントの情報を参照してください
カルタヘナ法
キットコンポーネントの情報を参照してください
Jan Code
キットコンポーネントの情報を参照してください
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
資料
Things to consider for preparation, setup and execution of the Duolink® assay protocol
Find Duolink references based on the type of method used, post translational modification detected, and research focus.
Find Duolink references based on the type of method used, post translational modification detected, and research focus.
Support information including tips and tricks, frequently asked questions, and basic troubleshooting.
プロトコル
Duolink® PLA Multicolor Detection Protocol
Duolink® PLA Multicolor Detection Protocol
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)