おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
sulindac
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
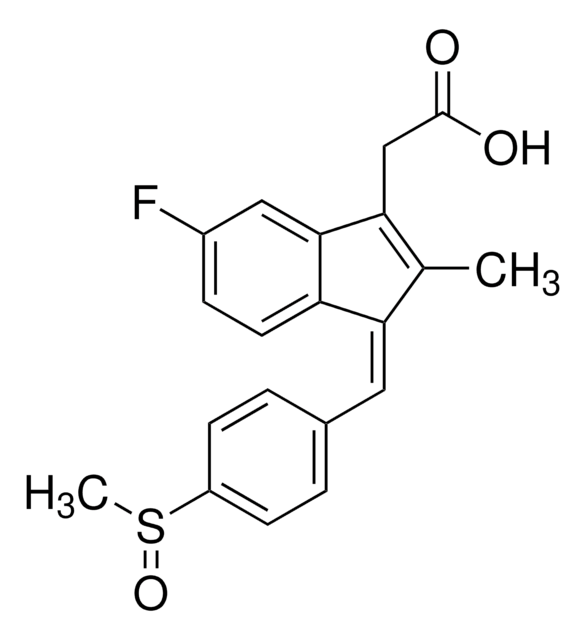
CC1=C(CC(O)=O)c2cc(F)ccc2\C1=C/c3ccc(cc3)S(C)=O
InChI
1S/C20H17FO3S/c1-12-17(9-13-3-6-15(7-4-13)25(2)24)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9-
InChI Key
MLKXDPUZXIRXEP-MFOYZWKCSA-N
遺伝子情報
human ... PTGS1(5742) , PTGS2(5743)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Sulindac EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生物化学的/生理学的作用
非ステロイド系抗炎症薬であり、COX-1の選択的インヒビタ-です。
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Oral - Repr. 2 - Resp. Sens. 1 - Skin Sens. 1
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
最新バージョンのいずれかを選択してください:
W R Waddell et al.
Journal of surgical oncology, 58(4), 252-256 (1995-04-01)
A putative explanation of the effect of sulindac on adenomatous colon and duodenal polyps from clinical observations and related in vitro experiments is presented. In cells with mutant APC genes, persistent high prostaglandin content of polyps leads to desensitization, downregulation
D E Duggan
Drug metabolism reviews, 12(2), 325-337 (1981-01-01)
Sulindac is a prodrug which, following absorption, rapidly attains a metabolic equilibrium with its active pharmacophore, the sulfide metabolite. At the level of the whole body, the reversible interconversion sulindac in equilibrium sulfide, and the differing distributional and excretory properties
R Andrew Moore et al.
The Cochrane database of systematic reviews, (4)(4), CD007540-CD007540 (2009-10-13)
Sulindac is a non-steroidal anti-inflammatory drug (NSAID) licensed for use in rheumatic disease and other musculoskeletal disorders in the UK, and widely available in other countries worldwide. This review sought to evaluate the efficacy and safety of oral sulindac in
Jigar Pravinchandra Modi et al.
Brain research, 1576, 91-99 (2014-06-27)
The present study analyzed whether administration of sulindac, a non-steroidal anti-inflammatory drug (NSAID) would prevent, attenuate or repair ischemia induced brain injury and reverse functional impairment in a focal ischemia model of stroke. Male Sprague-Dawley rats (weight 250-300 g) were
F M Giardiello
Cancer metastasis reviews, 13(3-4), 279-283 (1994-12-01)
Sulindac is useful in regression of adenomatous polyps. In addition to orally administered sulindac, rectal preparations also appear to be efficacious [32]. However, further studies are necessary to determine whether regression of adenomas, the precursor of colorectal cancer, will cause
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)