おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
rifamycin, rifampicin
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
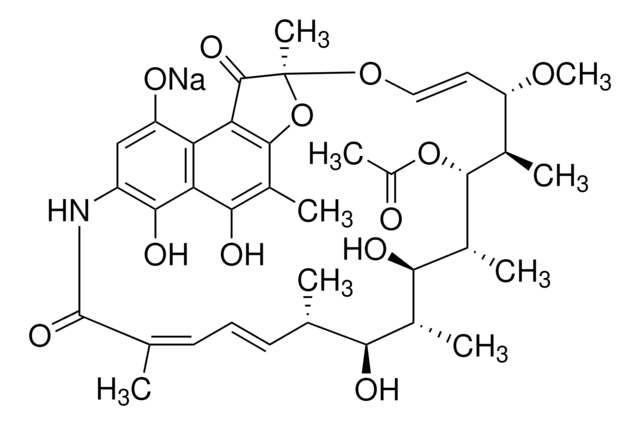
SMILES記法
N1c2c(c3c(c4c(c(c3O)C)O[C@](O\C=C\[C@@H]([C@H]([C@H]([C@@H]([C@@H]([C@@H]([C@H]([C@H](\C=C\C=C(/C1=O)\C)C)O)C)O)C)OC(=O)C)C)OC)(C4=O)C)c(c2)OCC(=O)O)O
InChI
1S/C39H49NO14/c1-17-11-10-12-18(2)38(49)40-24-15-26(51-16-27(42)43)28-29(34(24)47)33(46)22(6)36-30(28)37(48)39(8,54-36)52-14-13-25(50-9)19(3)35(53-23(7)41)21(5)32(45)20(4)31(17)44/h10-15,17,19-21,25,31-32,35,44-47H,16H2,1-9H3,(H,40,49)(H,42,43)/b11-10+,14-13+,18-12-/t17-,19+,20+,21+,25-,31-,32+,35+,39-/m0/s1
InChI Key
SQTCRTQCPJICLD-KTQDUKAHSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Rifamycin B EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Zhi-Hua Jin et al.
Journal of Zhejiang University. Science, 5(12), 1590-1596 (2004-11-18)
Study of the effect of dissolved oxygen and shear stress on rifamycin B fermentation with A. mediterranei XC 9-25 showed that rifamycin B fermentation with Amycolatoposis mediterranei XC 9-25 needs high dissolved oxygen and is not very sensitive to shearing
Zhi Hua Jin et al.
The Journal of general and applied microbiology, 48(6), 329-334 (2003-04-12)
An industrially applied rifamycin B-producing strain, Amycolatopsis mediterranei XC 1-02, was used for further screening. A special mutation and screening procedure was adopted to select a strain, which can alleviate the inhibition caused by both aromatic amino acid and p-hydroxybenzoic
Jun Xu et al.
Archives of biochemistry and biophysics, 411(2), 277-288 (2003-03-08)
The gene rif orf14 in the rifamycin biosynthetic gene cluster of Amycolatopsis mediterranei S699, producer of the antitubercular drug rifamycin B, encodes a protein of 272 amino acids identified as an AdoMet: 27-O-demethylrifamycin SV methyltransferase. Frameshift inactivation of rif orf14
Xuan-Tien Doan et al.
Journal of biotechnology, 132(2), 156-166 (2007-08-04)
Industrial production of antibiotics, biopharmaceuticals and enzymes is typically carried out via a batch or fed-batch fermentation process. These processes go through various phases based on sequential substrate uptake, growth and product formation, which require monitoring due to the potential
Prashant M Bapat et al.
Applied microbiology and biotechnology, 72(4), 662-670 (2006-03-15)
It is well-known that secondary metabolite production is repressed by excess nitrogen substrate available in the fermentation media. Although the nitrogen catabolite repression has been known, quantitative process models have not been reported to represent this phenomenon in complex medium.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)