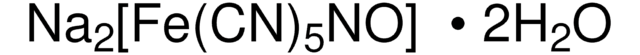おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to Ph. Eur. S2151000
traceable to USP 1632004
APIファミリー
sulfanilamide
CofA
current certificate can be downloaded
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-30°C
詳細
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards.
Sulfanilamide is an antibacterial drug.
アプリケーション
It was used in a study to demonstrate photodecomposition in aqueous solution of cutaneous photosensitizing agents with the help of spin traps 5, 5-dimethyl-1-pyrroline-1-oxide.
Sulfanilamide may be used as a pharmaceutical reference standard for the determination of the analyte in wastewater samples and biological fluids as well as pharmaceutical formulations by liquid chromatography-tandem mass spectrometry and spectrophotometry/chromatography, respectively.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
アナリシスノート
このような2次標準は、USP、EP(PhEur)、BPの1次標準にマルチトレーサビリティを提供します。
その他情報
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
脚注
To see an example of a Certificate of Analysis for this material enter LRAA9003 in the slot below. This is an example certificate only and may not be the lot that you receive.
関連製品
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1184-5G:
PHR1184-5G-PW:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
Determination of 76 pharmaceutical drugs by liquid chromatography--tandem mass spectrometry in slaughterhouse wastewater.
Shao B, et al.
Journal of Chromatography A, 1216(47), 8312-8318 (2009)
Spectrophotometric and chromatographic determination of sulfanilamides in biological fluids and pharmaceuticals.
Evgen?ev MI, et al.
Journal of Analytical Chemistry, 55(8), 799-805 (2000)
SPECTROSCOPIC STUDIES OF CUTANEOUS PHOTOSENSITIZING AGENTS?II. SPIN TRAPPING OF PHOTOLYSIS PRODUCTS FROM SULFANILAMIDE AND 4-AMINOBENZOIC ACID USING 5, 5-DIMETHYL-1-PYRROLINE-1-OXIDE.
Chignell, Colin F., et al.
Photochemistry and Photobiology, 34.2 , 147-156 (1981)
Indeewari Kalhari Silva et al.
Pharmacognosy magazine, 7(27), 193-199 (2011-10-05)
A decoction prepared with barks of Adenanthera pavonina and Thespesia populnea is a herbal formulation which has been prescribed in Sri Lanka in the treatment of cancer patients for many years. This study was designed to investigate its phytochemical and
Wenfa Lu et al.
Journal of parasitology research, 2011, 316067-316067 (2011-05-18)
Wild-type (WT) C57BL/6 mice infected intraperitoneally with 5 × 10(6) Trypanosoma congolense survive for more than 30 days. C57BL/6 mice deficient in inducible nitric oxide synthase (iNOS(-/-)) and infected with 10(3) or 5 × 10(6) parasites do not control the
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)