おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
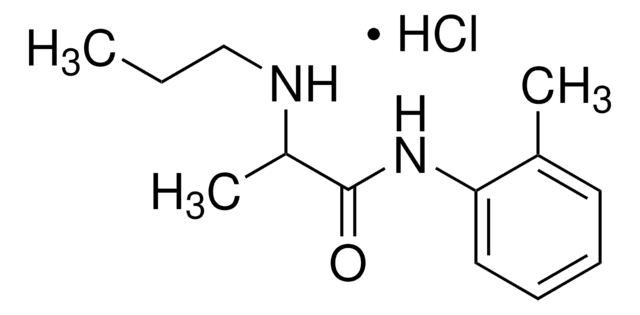
prilocaine
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
InChI
1S/C13H20N2O/c1-4-9-14-11(3)13(16)15-12-8-6-5-7-10(12)2/h5-8,11,14H,4,9H2,1-3H3,(H,15,16)
InChI Key
MVFGUOIZUNYYSO-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Prilocaine EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Eye Irrit. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 3
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
M Y Hyun et al.
Clinical and experimental dermatology, 40(2), 129-135 (2014-10-17)
Hyaluronic acid (HA) fillers and poly-L-lactic acid (PLA) fillers are frequently used to correct facial wrinkles. To compare the efficacy and safety of a novel injectable poly-L-lactic acid (PLA) filler and a well-studied biphasic HA filler for the treatment of
Elena I Gubanova et al.
Journal of drugs in dermatology : JDD, 14(3), 288-298 (2015-03-05)
In this evaluator-blind, placebo-controlled study, microinjections of stabilized hyaluronic acid (HA) gel for rejuvenation of aging hands were evaluated. Patients received three injections of 1.0 ml HA gel (20 mg/ml HA) in the dorsum of one hand and 1.0 ml
Murad Alam et al.
JAMA dermatology, 150(8), 844-849 (2014-06-13)
Neocollagenesis can be achieved using a dermal rolling needle device, thereby reducing the appearance of acne scars. To determine the efficacy of a needling device for treatment of acne scars. We performed a single-center, rater-blinded, balanced (1:1), split-face, placebo-controlled, parallel-group
Hee Jung Lee et al.
The Journal of dermatological treatment, 26(1), 73-77 (2014-02-12)
Intense focused ultrasound (IFUS) has been used successfully for skin tightening. To investigate the efficacy of IFUS in treating enlarged pores and to evaluate changes in skin elasticity and sebum production following IFUS. Twenty-two subjects with enlarged pores were randomized
Adam Rish
Journal of cosmetic dermatology, 13(4), 253-260 (2014-11-18)
A review of 242 facial treatments, in 86 different patients, with polyacrylamide gel (PAAG) was carried out by the author between 2003 and 2013. To evaluate by retrospective study the long-term outcomes of PAAG filling for facial contouring. To quantify
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)