おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
baclofen
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
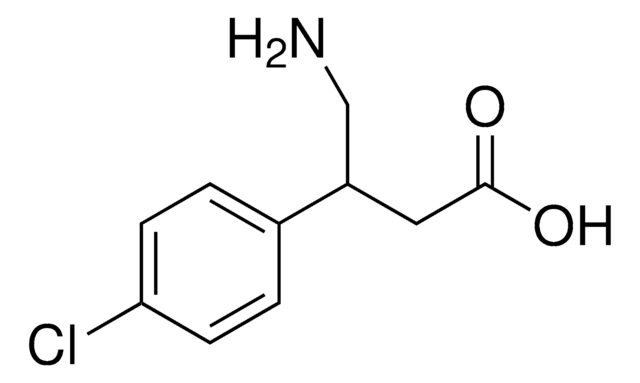
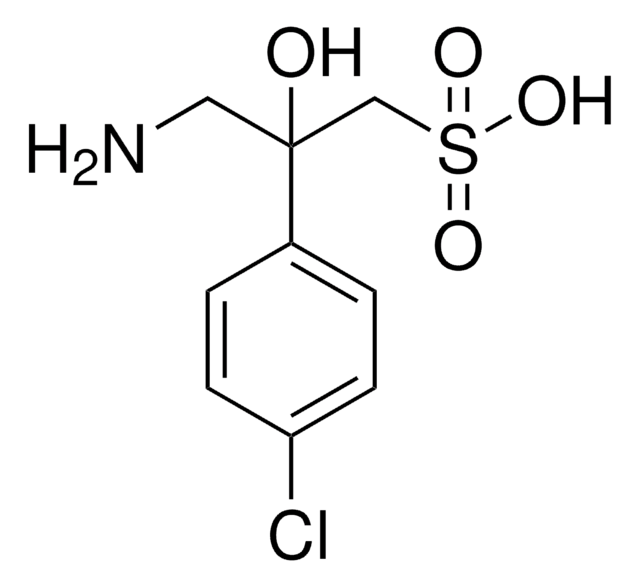
ClC1=CC=C(C(CN)CC(O)=O)C=C1
InChI
1S/C10H12ClNO2/c11-9-3-1-7(2-4-9)8(6-12)5-10(13)14/h1-4,8H,5-6,12H2,(H,13,14)
InChI Key
KPYSYYIEGFHWSV-UHFFFAOYSA-N
遺伝子情報
human ... GABBR1(2550) , GABBR2(9568)
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Baclofen EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生物化学的/生理学的作用
GABABレセプタ-のアゴニストであり、骨格筋弛緩剤、抗けいれん薬です。
包装
この製品は発行元の薬局方による供給としてお届けします。現在の単位量については、EDQM reference substance catalogueをご覧ください。
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Oral
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
B0200000:
B0200000-1EA:
B0200000-50MG:
最新バージョンのいずれかを選択してください:
A C Miracle et al.
AJNR. American journal of neuroradiology, 32(7), 1158-1164 (2010-10-30)
ITB pumps are widely used in the treatment of intractable spasticity for many clinical indications, including cerebral palsy and spinal cord injury. High-dose intrathecal administration places the patient at significant risk for withdrawal in the event of device malfunction, necessitating
Andrew J Muzyk et al.
CNS drugs, 26(1), 69-78 (2011-12-08)
The pharmacological properties of baclofen, a GABA(B) receptor agonist, have led to investigation of its use for the off-label treatment of alcohol dependence. Literature examining the role of baclofen in alcohol dependence suggests that it may be a useful medication
Laura A Bonouvrié et al.
European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society, 16(3), 279-284 (2011-10-22)
Intrathecal baclofen (ITB) treatment is frequently used for individuals with severe, but non-progressive, spasticity refractory to oral treatment. However, experiences with ITB in patients with progressive neurological disorders of childhood causing spasticity are limited. To investigate whether ITB is an
April Erwin et al.
Multiple sclerosis (Houndmills, Basingstoke, England), 17(5), 623-629 (2011-02-02)
The majority of patients with multiple sclerosis (MS) have symptoms of spasticity that increasingly impair function as the disease progresses. With appropriate treatment, however, quality of life can be improved. Oral antispasticity medications are useful in managing mild spasticity but
Amanda McIntyre et al.
The journal of spinal cord medicine, 37(1), 11-18 (2013-10-05)
To review the available evidence on the effectiveness of intrathecal baclofen in the treatment of spasticity in individuals with spinal cord injuries (SCIs) at least 6 months post-injury or diagnosis. A literature search of multiple databases (Pub Med, CINAHL, EMBASE)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)