MABN1839
Anti-Amyloid-β (oligomer) Antibody, clone F11G3
clone F11G3, from mouse
別名:
Amyloid beta oligomer, Abeta oligomer, alpha-synuclein oligomer,, alpha-syn oligomer, Prion protein oligomer, PrP oligomer, TAR DNA-binding protein 43 oligomer, TDP-43 oligomer, Tau oligomer
About This Item
おすすめの製品
由来生物
mouse
品質水準
抗体製品の状態
purified immunoglobulin
抗体製品タイプ
primary antibodies
クローン
F11G3, monoclonal
化学種の反応性
all, human, mouse
テクニック
ELISA: suitable
dot blot: suitable
immunofluorescence: suitable
immunoprecipitation (IP): suitable
western blot: suitable
アイソタイプ
IgMκ
NCBIアクセッション番号
UniProtアクセッション番号
輸送温度
dry ice
ターゲットの翻訳後修飾
unmodified
遺伝子情報
human ... APP(351) , PRNP(5621) , SNCA(6622) , TARDBP(23435)
詳細
特異性
免疫原
アプリケーション
ニューロサイエンス
神経変性疾患
Western Blotting Analysis: A representative lot detected Ataxin-1 oligomers in soluble cerebella extracts from Atxn1154Q/+, but not wild-type or Atxn-/-, mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Western Blotting Analysis: A representative lot detected cellular beta-sheet oligomer immunoreactivity in HeLa cells transfected with the pathogenic (82Q), but not the non-pathogenic (30Q) form of polyQ Ataxin-1 in transfected Hela cells. Co-transfecting with the native Atxn-1 binding partner Capicua (CIC), but not the binding defective CIC W37A mutant, enhanced the oligomer formation (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Western Blotting Analysis: A representative lot detected the highest extend of oligomers accumulation in the soluble cerebella extracts among the 28-week old Atxn1154Q/+ mice when compared with samples from 18-week old and 8-week old Atxn1154Q/+ mice, with the 8-week old mice bearing the least oligomer buildup (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
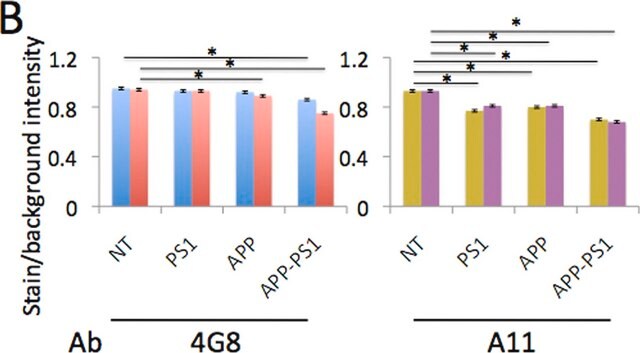
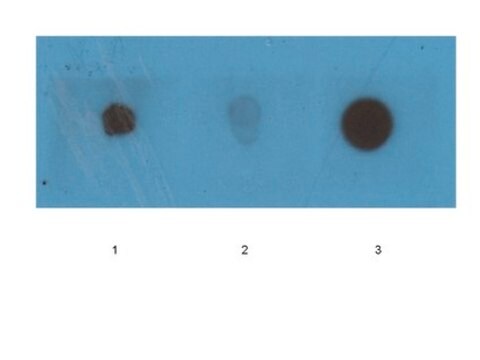
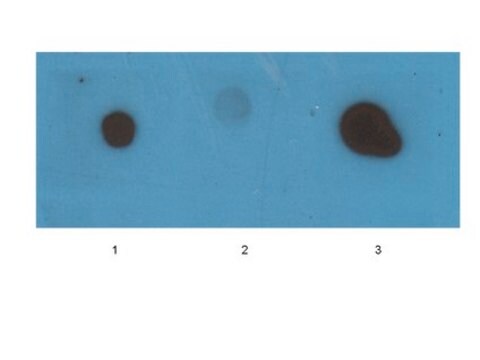
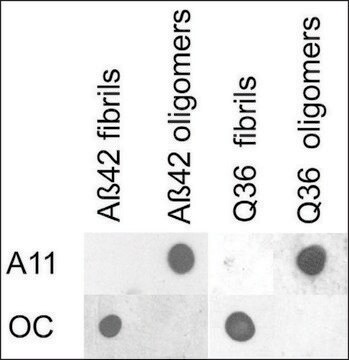
Western Blotting Analysis: A representative lot specifically detected oligomeric, but not monomeric or fibrillar, forms of Aβ42, α-Syn, PrP, and TDP-43 (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
App3/DB/ A representative lot specifically detected oligomeric, but not monomeric or fibrillar, forms of Aβ42, α-Syn, PrP, and TDP-43 (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
ELISA Analysis: A representative lot detected in vitro Aβ42, α-Syn, PrP, and TDP-43 oligomers formation with or without Aβ42 oligomer seeding (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
Immunofluorescence Analysis: A representative lot detected a positive correlation between the ATXN1 beta-sheet oligomer immunoreactivity and the degeneration progression in calbindin-positive Purkinje cells (PCs) by fluorescent immunohistochemistry using paraffin-embedded cerebellum sections from Atxn1154Q/+ mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Immunofluorescence Analysis: A representative lot selectively detected beta-sheet oligomer immunoreactivity colocalized with those of Aβ, α-Syn, PrP, and TDP-43 in paraffin-embedded frontal cortex sections from Alzheimer′s diseased brain by fluorescent immunohistochemistry. The beta-sheet oligomer immunoreactivity is not detected in non-AD brains and is distinct from the staining pattern obtained with Thioflavin S (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
Immunoprecipitation Analysis: A representative lot immunoprecipitated Ataxin-1 oligomers from the soluble cerebella extracts of Atxn1154Q/+, but not Atxn-/-, mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Immunocytochemistry Analysis: A representative lot detected cellular beta-sheet oligomer immunoreactivity in HeLa cells transfected with the pathogenic polyQ Ataxin-1 mRFP fusion construct mRFP-ATXN1(82Q) by fluorescent immunocytochemistry. Co-transfecting with the N-terminal fragment of the Atxn-1 binding partner Capicua (CIC), but not the binding defective CIC W37A mutant fragment, enhanced the oligomer formation (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
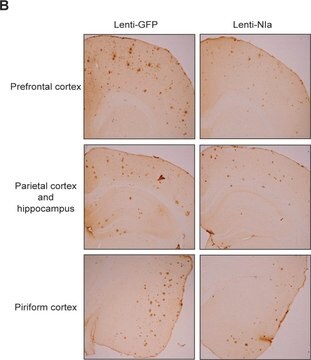
Immunohistochemistry Analysis: A representative lot detected beta-sheet oligomer immunoreactivity in paraffin-embedded cerebellum and cortex sections of Atxn1154Q/+, but not wild-type, mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
品質
Western Blotting Analysis: 2.0 µg/mL of this antibody detected 10 µg of oligomeric amyloid.
ターゲットの説明
物理的形状
保管および安定性
Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze/thaw cycles, which may damage IgG and affect product performance.
その他情報
免責事項
適切な製品が見つかりませんか。
製品選択ツール.をお試しください
保管分類コード
12 - Non Combustible Liquids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
MABN1839:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)