おすすめの製品
化学種の反応性
human
メーカー/製品名
Milliplex®
テクニック
multiplexing: suitable
assay range
inter-assay cv: <20%
intra-assay cv: <10%
検出方法
fluorometric (Luminex® xMAP®)
保管温度
2-8°C
詳細
Cancer autoantibodies have been studied as reporters of early carcinogenesis and indicators of cancer prognosis. Autoantibodies to tumor-associated antigens are putative cancer biomarkers, as these autoantibodies are abundant, detectable in early stages, and stable in serum. These autoantibodies may be developed from humoral immune responses to the changes in the expression levels of tumor-associated self-antigens, misfolded proteins, mutant antigens, and proteins involved in inflammation and the cellular death process. While the frequency of cancer autoantibody responses to an individual tumor antigen is typically low, the response to a multiplexed panel of antigens could be much higher. An optimized multiplex autoantibody panel to common tumor-associated antigens can improve the potential of using autoantibodies as biomarkers to study cancer development and progression, and it can serve as a simple and affordable tool in evaluating large numbers of patient samples for multiple cancer types.
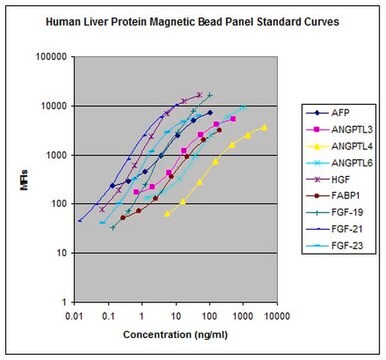
The MILLIPLEX® MAP Human Cancer Autoantibody Magnetic Bead Panel is a 15-plex kit to be used for the simultaneous quantification of autoantibodies to any or all of the following analytes in serum and plasma samples: Alpha-enolase (ENO1), Cancer/Testis Antigen 1B (CTAG1B/NY-ESO-1), Cyclin-dependent kinase 4 inhibitor A (p16-INK4a), G2/mitotic-specific cyclin-B1 (CCNB1), Galactose-specific lectin-1 (Galectin 1), Galactose-specific lectin-3 (Galectin 3), Heat shock protein 60 (HSP60), Hypoxia-inducible factor 1-alpha (HIF1α), Insulin-like growth factor 2 mRNA-binding protein 2 (IMP-2), Insulin-like growth factor 2 mRNA-binding protein 3 (IMP-3), Mucin 1 (MUC1), Receptor tyrosine-protein kinase (HER2/ErbB2), sex determining region Y (SRY)-box 2 (SOX2), Survivin (Survivin/BIRC5), and Tumor protein p53 (p53). For this particular panel, four assay control beads are included as part of the base kit format.
The MILLIPLEX® MAP Human Cancer Autoantibody Magnetic Bead Panel is a 15-plex kit to be used for the simultaneous quantification of autoantibodies to any or all of the following analytes in serum and plasma samples: Alpha-enolase (ENO1), Cancer/Testis Antigen 1B (CTAG1B/NY-ESO-1), Cyclin-dependent kinase 4 inhibitor A (p16-INK4a), G2/mitotic-specific cyclin-B1 (CCNB1), Galactose-specific lectin-1 (Galectin 1), Galactose-specific lectin-3 (Galectin 3), Heat shock protein 60 (HSP60), Hypoxia-inducible factor 1-alpha (HIF1α), Insulin-like growth factor 2 mRNA-binding protein 2 (IMP-2), Insulin-like growth factor 2 mRNA-binding protein 3 (IMP-3), Mucin 1 (MUC1), Receptor tyrosine-protein kinase (HER2/ErbB2), sex determining region Y (SRY)-box 2 (SOX2), Survivin (Survivin/BIRC5), and Tumor protein p53 (p53). For this particular panel, four assay control beads are included as part of the base kit format.
アプリケーション
- Analytes Available: CCNB1/Cyclin B1, CTAG1B/NY-ESO-1, ENO1, Galectin-1 (GAL1), Galectin-3, ErbB2/HER2, HIF-1α, HSP60, IGF2BP2/IMP2, IGF2BP3/IMP3, Mucin-1 (MUC1), P16-INK4a, p53, Sox2, Survivin/BIRC5.
- Panel Type: MAGNETIC Circulating Cancer
- This is assay yields results in Median Fluorescence Intensity (MFI).
- Four assay control beads are included in the base kit.
- This is an overnight assay.
- Recommended dilution: This assay requires 25 μL of 1:100 diluted plasma or serum.
- Research Category: Oncology
- Research Sub-Category: Oncology, Tumor Markers, Immune Response
特徴および利点
Design your multiplex kit by choosing available analytes within this panel.
保管および安定性
Recommended storage for kit components is 2 - 8°C.
法的情報
Luminex is a registered trademark of Luminex Corp
MILLIPLEX is a registered trademark of Merck KGaA, Darmstadt, Germany
xMAP is a registered trademark of Luminex Corp
免責事項
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Sens. 1
保管分類コード
12 - Non Combustible Liquids
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
毒物及び劇物取締法
キットコンポーネントの情報を参照してください
PRTR
キットコンポーネントの情報を参照してください
消防法
キットコンポーネントの情報を参照してください
労働安全衛生法名称等を表示すべき危険物及び有害物
キットコンポーネントの情報を参照してください
労働安全衛生法名称等を通知すべき危険物及び有害物
キットコンポーネントの情報を参照してください
カルタヘナ法
キットコンポーネントの情報を参照してください
Jan Code
キットコンポーネントの情報を参照してください
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)