おすすめの製品
物質
PureFlex™ polyethylene bag (film)
polyester body
silicone septum (platinum-cured)
silicone tubing
stainless steel body (ASTM® 316 L)
品質水準
認証
according to ISO ISO 11137
meets USP 88 Biological Reactivity
無菌性
sterile; β-irradiated
製品種目
NovaSeptum® GO
特徴
autoclavable
パラメーター
-20-125 °C temp. range (-4-257 °F)
0.30 bar max. pressure (4.35 psi)
不純物
<2.15 bacterial endotoxins (LAL test, EU/device)
フィッティング
male Luer-Lok® outlet (with female sterile vent filter)
アプリケーション
cell therapy
gene therapy
life science and biopharma
mAb
ophthalmics
parenterals
pharma/biopharma processes
sterile sampling
viral therapy
保管温度
room temp
詳細
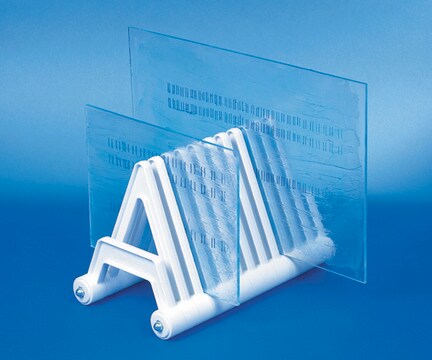
A range of NovaSeptum® GO small pack size kits available to trial a sterile sampling solution for your process. Choose from several collection container types: high purity bags, autoclavable bags, bottles or accurate volume syringes (5 containers per pack). The closed, sterile sampling method can significantly reduce risk of cross contamination. Ideal for sampling from aseptic and sterile processes, the NovaSeptum® GO sterile sampling system provides consistently representative samples, safely and securely. NovaSeptum® GO Accurate Volume Syringes are designed to take samples from 1 mL to 20 mL, enabling operators to directly dispense sample products with milliliter accuracy. Autoclavable bags are intended for applications where the unit is connected to a vessel that needs to be sterilized by autoclave. Bio-neutral plastic bottles or high purity bags are ideal for all tests and are available in a range of sizes. Use a trial kit in combination with a NovaSeptum® GO holder.
アプリケーション
Sterile sampling
Sterility testing, pyrogenic testing, endotoxin testing, chemical analysis, pH analysis, and fermentation applications
包装
5x autoclavable bag 250ml
構成
- Septum: Platinum-cured silicone; Body: Polyester
- Cannula: ASTM® 316 L Stainless steel
- Fluid contact layer (bag): Polypropylene film
- Tubing: Silicone
- Outlet Tubing: Male Luer-Lok® with female sterile vent filter
調製ノート
All component materials, in contact with sampling liquid, meet the criteria for Class VI testing based on USP <88> Biological Reactivity, in vivo.
Units are integrity tested at regular intervals during manufacturing.
Aqueous extraction contains < 2.15 EU per device as determined using the Limulus Amebocyte Lysate (LAL) test.
Units are integrity tested at regular intervals during manufacturing.
Aqueous extraction contains < 2.15 EU per device as determined using the Limulus Amebocyte Lysate (LAL) test.
Assembled under ISO Clean Room Class 8 conditions in a facility certified to ISO 14644-1 standards.
法的情報
ASTM is a registered trademark of American Society for Testing and Materials
Luer-Lok is a registered trademark of Becton-Dickinson & Co.
NOVASEPTUM is a registered trademark of Merck KGaA, Darmstadt, Germany
PUREFLEX is a trademark of Merck KGaA, Darmstadt, Germany
適切な製品が見つかりませんか。
製品選択ツール.をお試しください
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)