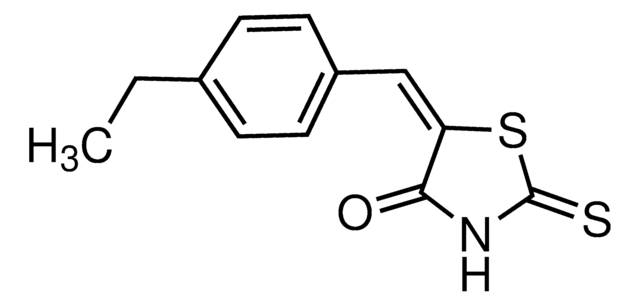
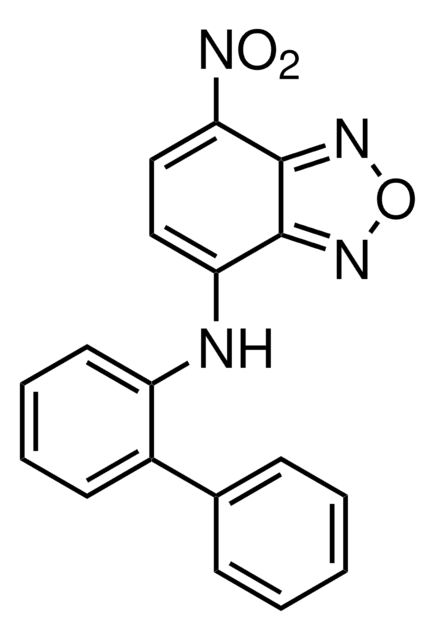
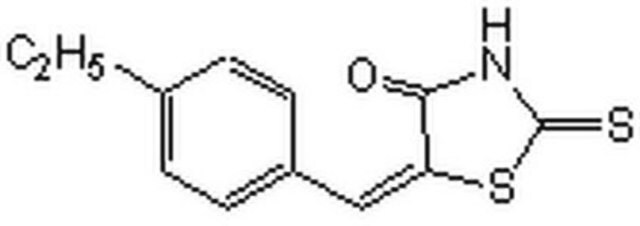
475957
c-Myc Inhibitor II
The c-Myc Inhibitor II, also referenced under CAS 413611-93-5, controls the biological activity of c-Myc.
別名:
c-Myc Inhibitor II, 7-Nitro-N-(2-phenylphenyl)-2,1,3-benzoxadiazol-4-amine, Biphenyl-2-yl-(7-nitrobenzo[1,2,5]oxadiazol-4-ylamine, 10074-G5
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
おすすめの製品
品質水準
アッセイ
≥95% (HPLC)
フォーム
solid
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
protect from light
色
deep orange
溶解性
DMSO: 50 mg/mL
輸送温度
ambient
保管温度
2-8°C
InChI
1S/C18H12N4O3/c23-22(24)16-11-10-15(17-18(16)21-25-20-17)19-14-9-5-4-8-13(14)12-6-2-1-3-7-12/h1-11,19H
InChI Key
KMJPYSQOCBYMCF-UHFFFAOYSA-N
詳細
A cell-permeable benzoxadiazole compound that is shown to preferentially disrupt the interactions of c-Myc-Max, Mad1-Max, and Myf5-HEB, over those of 31 other pairs of HLH-, ZIP-, and HLH-ZIP-containing proteins. Similarly to 10058-F4 (Cat. No. 475956), 10074-G5 is shown to selectively inhibit the c-Myc-dependent growth of rat fibroblast cell line TGR1 and effectively suppress c-Myc-dependent transcription activity. Binding studies using various mutated and truncated c-Myc constructs reveal that 10074-G5 targets c-Myc helix-1 region between aa residues 363 and 381 (KD = 4.4 µM), while 10058-F4 interaction site is located within aa residues 402 through 412 (KD = 13 µM).
A cell-permeable benzoxadiazole compound that is shown to preferentially disrupt the interactions of c-Myc-Max, Mad1-Max, and Myf5-HEB, over those of 31 other pairs of HLH-, ZIP-, and HLH-ZIP-containing proteins. Similarly to 10058-F4 (Cat. No. 475956), 10074-G5 is shown to selectively inhibit the c-Myc-dependent growth of rat fibroblast cell line TGR1 and effectively suppress c-Myc-dependent transcription activity. Binding studies using various mutated and truncated c-Myc constructs reveal that 10074-G5 targets c-Myc helix-1 region between aa 363 and 381 (KD = 4.4 µM), while 10058-F4 interaction site is located within aa residues 402 through 412 (KD = 13 µM).
包装
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
再構成
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
その他情報
Follis, A.V., et al. 2008. Chem. Biol.15, 1149.
Yin, X., et al. 2003. Oncogene22, 6151.
Sold under license of U.S. Patent 7,026,343.
Yin, X., et al. 2003. Oncogene22, 6151.
Sold under license of U.S. Patent 7,026,343.
法的情報
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
475957-10MG:
475957-MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Yue-Zhi Lee et al.
Pharmacological research, 152, 104581-104581 (2019-12-04)
Interruption of the Warburg effect - the observation that un-stimulated macrophages reprogram their core metabolism from oxidative phosphorylation toward aerobic glycolysis to become pro-inflammatory M1 macrophages upon stimulation - is an emerging strategy for the treatment of cancer and anti-inflammatory
Batuhan Mert Kalkan et al.
Microvascular research, 130, 104001-104001 (2020-03-22)
Endothelial dysfunction is prominent in atherosclerosis, hypertension, diabetes, peripheral and cardiovascular diseases, and stroke. Novel therapeutic approaches to these conditions often involve development of tissue-engineered veins with ex vivo expanded endothelial cells. However, high cell number requirements limit these approaches
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)