17-10188
LentiBrite RFP-LC3 Control Mutant Lentiviral Biosensor
別名:
Microtubule-associated proteins 1A/1B light chain 3, Autophagy-related protein LC3, Autophagy-related ubiquitin-like modifier LC3, MAP1A/MAP1B light chain 3, Microtubule-associated protein 1 light chain 3
ログイン組織・契約価格を表示する
すべての画像(3)
About This Item
UNSPSCコード:
12352207
eCl@ss:
34360190
NACRES:
NA.51
おすすめの製品
詳細
Read our application note in Nature Methods!
http://www.nature.com/app_notes/nmeth/2012/121007/pdf/an8620.pdf
(Click Here!)
Learn more about the advantages of our LentiBrite Lentiviral Biosensors! Click Here
Biosensors can be used to detect the presence/absence of a particular protein as well as the subcellular location of that protein within the live state of a cell. Fluorescent tags are often desired as a means to visualize the protein of interest within a cell by either fluorescent microscopy or time-lapse video capture. Visualizing live cells without disruption allows researchers to observe cellular conditions in real time.
Lentiviral vector systems are a popular research tool used to introduce gene products into cells. Lentiviral transfection has advantages over non-viral methods such as chemical-based transfection including higher-efficiency transfection of dividing and non-dividing cells, long-term stable expression of the transgene, and low immunogenicity.
EMD Millipore is introducing LentiBrite Lentiviral Biosensors, a new suite of pre-packaged lentiviral particles encoding important and foundational proteins of autophagy, apoptosis, and cell structure for visualization under different cell/disease states in live cell and in vitro analysis.
• Pre-packaged, fluorescently-tagged with GFP & RFP
• Higher efficiency transfection as compared to traditional chemical-based and other non-viral-based transfection methods
• Ability to transfect dividing, non-dividing, and difficult-to-transfect cell types, such as primary cells or stem cells
•Non-disruptive towards cellular function
EMD Millipore’s LentiBrite RFP-LC3-G120A lentiviral particles serve as a negative control alongside RFP-LC3 wild-type (catalog # 17-10143) for live cell analysis of autophagy.
http://www.nature.com/app_notes/nmeth/2012/121007/pdf/an8620.pdf
(Click Here!)
Learn more about the advantages of our LentiBrite Lentiviral Biosensors! Click Here
Biosensors can be used to detect the presence/absence of a particular protein as well as the subcellular location of that protein within the live state of a cell. Fluorescent tags are often desired as a means to visualize the protein of interest within a cell by either fluorescent microscopy or time-lapse video capture. Visualizing live cells without disruption allows researchers to observe cellular conditions in real time.
Lentiviral vector systems are a popular research tool used to introduce gene products into cells. Lentiviral transfection has advantages over non-viral methods such as chemical-based transfection including higher-efficiency transfection of dividing and non-dividing cells, long-term stable expression of the transgene, and low immunogenicity.
EMD Millipore is introducing LentiBrite Lentiviral Biosensors, a new suite of pre-packaged lentiviral particles encoding important and foundational proteins of autophagy, apoptosis, and cell structure for visualization under different cell/disease states in live cell and in vitro analysis.
• Pre-packaged, fluorescently-tagged with GFP & RFP
• Higher efficiency transfection as compared to traditional chemical-based and other non-viral-based transfection methods
• Ability to transfect dividing, non-dividing, and difficult-to-transfect cell types, such as primary cells or stem cells
•Non-disruptive towards cellular function
EMD Millipore’s LentiBrite RFP-LC3-G120A lentiviral particles serve as a negative control alongside RFP-LC3 wild-type (catalog # 17-10143) for live cell analysis of autophagy.
Autophagy, a stress-induced degradative pathway, plays a role in many diseases, including cancer, neurodegeneration, and infections. Members of the LC3 family play a key role in the maturation of the autophagosome, the central organelle of autophagy. LC3 precursors are proteolytically processed to form cytosolic LC3-I. Upon initiation of autophagy, the C-terminal glycine is modified by addition of phosphatidylethanolamine (PE) to form LC3-II, which translocates to autophagosomes in a punctate distribution. DNA constructs encoding fluorescent proteins fused to LC3 are widely employed for monitoring autophagosome formation by fluorescence microscopy.
A mutant form of LC3 with the C-terminal glycine changed to alanine (LC3-G120A) is unable to accept the PE modification and fails to translocate to the autophagosome upon induction of autophagy.
EMD Millipore’s LentiBrite RFP-LC3-G120A lentiviral particles serve as a negative control alongside RFP-LC3 wild-type (catalog # 17-10143) for live cell analysis of autophagy.
A mutant form of LC3 with the C-terminal glycine changed to alanine (LC3-G120A) is unable to accept the PE modification and fails to translocate to the autophagosome upon induction of autophagy.
EMD Millipore’s LentiBrite RFP-LC3-G120A lentiviral particles serve as a negative control alongside RFP-LC3 wild-type (catalog # 17-10143) for live cell analysis of autophagy.
アプリケーション
Research Category
アポトーシス及び癌
ニューロサイエンス
アポトーシス及び癌
ニューロサイエンス
Research Sub Category
アポトーシス-追加
神経変性疾患
アポトーシス-追加
神経変性疾患
Fluorescence Microscopy Imaging:
(See Figure 1 in datasheet)
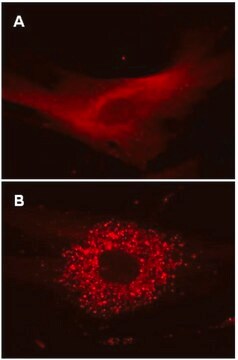
HT-1080 cells were plated in a chamber slide and transduced with lentiviral particles at an MOI of 20 for 24 hours. After media replacement and 48 hours further incubation, cells were either left in complete media or incubated for 4 hours in EBSS containing a lysosome inhibitor, to induce autophagy and inhibit lysosomal degradation of autophagosomes. Cells were fixed with formaldehyde and mounted. Images were obtained by oil immersion wide-field fluorescence microscopy. The RFP-LC3 Control Mutant displays a diffuse nuclear and cytosolic distribution in both fed and starved autophagic cells (i.e., no translocation to a punctate cytoplasmic distribution as characteristic of wild-type LC3).
Immunocytochemistry Comparison and Inhibitor Analysis:
(See Figure 2 in datasheet)
Similar to Figure 1 (see datasheet), HeLa cells were plated in a chamber slide and transduced with lentiviral particles at an MOI of 20 for 24 hours. After media replacement and 48 hours further incubation, cells were either left in complete media, incubated for 4 hours in EBSS containing a lysosome inhibitor to induce autophagy and inhibit lysosomal degradation, or incubated as in, with the addition of 5 mM 3-methyladenine (3-MA) as an inhibitor of autophagy. 3-MA does not affect RFP-LC3 Control Mutant localization. Immunocytochemical staining (green) of the same fields of view with a monoclonal antibody against LC3A reveals a similar expression pattern to the mutant protein (red) under fed conditions. Immunostaining of starved cells displays the punctate distribution of endogenous wild-type LC3, while signal following 3-MA treatment is diminished.
Hard-to-transfect Cell Types:
(See Figure 3 in datasheet)
Primary cell types HUVEC (top row) or HuMSC (bottom row) were plated in chamber slides and transduced with lentiviral particles at an MOI of 40 for 24 hours. Subsequent treatments for cells left in complete media or cells incubated in EBSS with lysosome inhibitor, were performed as in Figures 1A and 1B (see datasheet).
For optimal fluorescent visualization, it is recommended to analyze the target expression level within 24-48 hrs after transfection/infection for optimal live cell analysis, as fluorescent intensity may dim over time, especially in difficult-to-transfect cell lines. Infected cells may be frozen down after successful transfection/infection and thawed in culture to retain positive fluorescent expression beyond 24-48 hrs. Length and intensity of fluorescent expression varies between cell lines. Higher MOIs may be required for difficult-to-transfect cell lines.
(See Figure 1 in datasheet)
HT-1080 cells were plated in a chamber slide and transduced with lentiviral particles at an MOI of 20 for 24 hours. After media replacement and 48 hours further incubation, cells were either left in complete media or incubated for 4 hours in EBSS containing a lysosome inhibitor, to induce autophagy and inhibit lysosomal degradation of autophagosomes. Cells were fixed with formaldehyde and mounted. Images were obtained by oil immersion wide-field fluorescence microscopy. The RFP-LC3 Control Mutant displays a diffuse nuclear and cytosolic distribution in both fed and starved autophagic cells (i.e., no translocation to a punctate cytoplasmic distribution as characteristic of wild-type LC3).
Immunocytochemistry Comparison and Inhibitor Analysis:
(See Figure 2 in datasheet)
Similar to Figure 1 (see datasheet), HeLa cells were plated in a chamber slide and transduced with lentiviral particles at an MOI of 20 for 24 hours. After media replacement and 48 hours further incubation, cells were either left in complete media, incubated for 4 hours in EBSS containing a lysosome inhibitor to induce autophagy and inhibit lysosomal degradation, or incubated as in, with the addition of 5 mM 3-methyladenine (3-MA) as an inhibitor of autophagy. 3-MA does not affect RFP-LC3 Control Mutant localization. Immunocytochemical staining (green) of the same fields of view with a monoclonal antibody against LC3A reveals a similar expression pattern to the mutant protein (red) under fed conditions. Immunostaining of starved cells displays the punctate distribution of endogenous wild-type LC3, while signal following 3-MA treatment is diminished.
Hard-to-transfect Cell Types:
(See Figure 3 in datasheet)
Primary cell types HUVEC (top row) or HuMSC (bottom row) were plated in chamber slides and transduced with lentiviral particles at an MOI of 40 for 24 hours. Subsequent treatments for cells left in complete media or cells incubated in EBSS with lysosome inhibitor, were performed as in Figures 1A and 1B (see datasheet).
For optimal fluorescent visualization, it is recommended to analyze the target expression level within 24-48 hrs after transfection/infection for optimal live cell analysis, as fluorescent intensity may dim over time, especially in difficult-to-transfect cell lines. Infected cells may be frozen down after successful transfection/infection and thawed in culture to retain positive fluorescent expression beyond 24-48 hrs. Length and intensity of fluorescent expression varies between cell lines. Higher MOIs may be required for difficult-to-transfect cell lines.
構成
TagRFP-LC3-G120A Lentivirus:
One vial containing 25 µL of lentiviral particles at a minimum of 3 x 10E8 infectious units (IFU) per mL.
For lot-specific titer information, please see lot specific “Viral Titer” in the product specifications of the datasheet.
Promoter
EF-1 (Elongation Factor-1)
Multiplicty of Infection (MOI)
MOI = Ratio of # of infectious lentiviral particles (IFU) to # of cells being infected.
Typical MOI values for high transduction efficiency and signal intensity are in the range of 20-40. For this target, some cell types may require lower MOIs (e.g., HT-1080, HeLa), while others may require higher MOIs (e.g., human umbilical vein endothelial cells (HUVEC), human mesenchymal stem cells (HuMSC), U2OS).
NOTE: MOI should be titrated and optimized by the end user for each cell type and lentiviral target to achieve desired transduction efficiency and signal intensity.
One vial containing 25 µL of lentiviral particles at a minimum of 3 x 10E8 infectious units (IFU) per mL.
For lot-specific titer information, please see lot specific “Viral Titer” in the product specifications of the datasheet.
Promoter
EF-1 (Elongation Factor-1)
Multiplicty of Infection (MOI)
MOI = Ratio of # of infectious lentiviral particles (IFU) to # of cells being infected.
Typical MOI values for high transduction efficiency and signal intensity are in the range of 20-40. For this target, some cell types may require lower MOIs (e.g., HT-1080, HeLa), while others may require higher MOIs (e.g., human umbilical vein endothelial cells (HUVEC), human mesenchymal stem cells (HuMSC), U2OS).
NOTE: MOI should be titrated and optimized by the end user for each cell type and lentiviral target to achieve desired transduction efficiency and signal intensity.
品質
Evaluated by transduction of HT-1080 cells and fluorescent imaging performed for assessment of transduction efficiency.
物理的形状
PEG precipitation
保管および安定性
Storage and Handling
Lentivirus is stable for at least 4 months from date of receipt when stored at -80°C. After first thaw, place immediately on ice and freeze in working aliquots at -80°C. Frozen aliquots may be stored for at least 2 months. Further freeze/thaws may result in decreased virus titer and transduction efficiency.
IMPORTANT SAFETY NOTE
Replication-defective lentiviral vectors, such as the 3rd Generation vector provided in this product, are not known to cause any diseases in humans or animals. However, lentiviruses can integrate into the host cell genome and thus pose some risk of insertional mutagenesis. Material is a Risk Group 2 and should be handled under BSL2 controls. A detailed discussion of biosafety of lentiviral vectors is provided in Pauwels, K. et al. (2009). State-of-the-art lentiviral vectors for research use: Risk assessment and biosafety recommendations. Curr. Gene Ther. 9: 459-474.
Lentivirus is stable for at least 4 months from date of receipt when stored at -80°C. After first thaw, place immediately on ice and freeze in working aliquots at -80°C. Frozen aliquots may be stored for at least 2 months. Further freeze/thaws may result in decreased virus titer and transduction efficiency.
IMPORTANT SAFETY NOTE
Replication-defective lentiviral vectors, such as the 3rd Generation vector provided in this product, are not known to cause any diseases in humans or animals. However, lentiviruses can integrate into the host cell genome and thus pose some risk of insertional mutagenesis. Material is a Risk Group 2 and should be handled under BSL2 controls. A detailed discussion of biosafety of lentiviral vectors is provided in Pauwels, K. et al. (2009). State-of-the-art lentiviral vectors for research use: Risk assessment and biosafety recommendations. Curr. Gene Ther. 9: 459-474.
法的情報
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
10 - Combustible liquids
WGK
WGK 2
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
17-10188:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
資料
High titer lentiviral particles for LC3 variants used for live cell analysis of cellular autophagy.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)