おすすめの製品
詳細
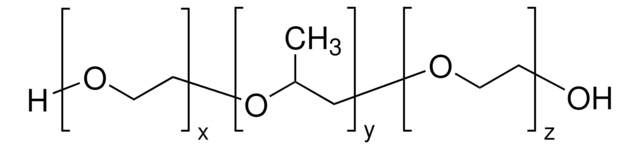
Detergents, such as non-ionic surfactants, are used in biomanufacturing processes for a variety of applications including viral inactivation and cell lysis. Detergent-mediated viral inactivation is widely used in multiple biotherapeutic production processes to ensure meeting regulatory requirements for viral safety.
Our SAFC portfolio of high-quality raw materials for use in biopharmaceutical processing withstands strict quality control procedures plus the documentation and expertise to help our customers meet requirements as defined by the M-Clarity Program.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
We offer a range of high quality critical raw materials plus the documentation and expertise to help our customers meet stringent regulatory requirements. Our SAFC portfolio of high-quality products withstands strict quality control procedures.
Our SAFC portfolio of high-quality raw materials for use in biopharmaceutical processing withstands strict quality control procedures plus the documentation and expertise to help our customers meet requirements as defined by the M-Clarity Program.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
We offer a range of high quality critical raw materials plus the documentation and expertise to help our customers meet stringent regulatory requirements. Our SAFC portfolio of high-quality products withstands strict quality control procedures.
アプリケーション
REACH compliantdetergent used in viral inactivation or cell lysis.
包装
1.08694.1000 / 1L / Glass bottle
1.08694.2500 / 2.5L / Glass bottle
1.08694.9025 / 25L / PE container
1.08694.2500 / 2.5L / Glass bottle
1.08694.9025 / 25L / PE container
法的情報
DEVIRON is a registered trademark of Merck KGaA, Darmstadt, Germany
Emprove is a registered trademark of Merck KGaA, Darmstadt, Germany
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2
保管分類コード
10 - Combustible liquids
WGK
WGK 1
引火点(°F)
469.4 °F - open cup
引火点(℃)
243 °C - open cup
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
第四石油類
危険等級III
労働安全衛生法名称等を表示すべき危険物及び有害物
名称等を表示すべき危険物及び有害物
労働安全衛生法名称等を通知すべき危険物及び有害物
名称等を通知すべき危険物及び有害物
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
関連コンテンツ
Learn more on mAb downstream processing, more specifically virus inactivation and the relevant associated products.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)