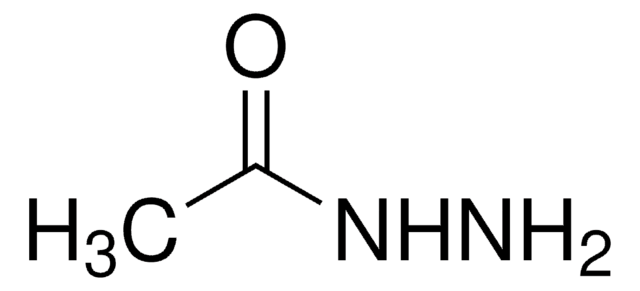
おすすめの製品
アッセイ
98%
形状
powder
bp
209 °C/15 mmHg (lit.)
mp
138-140 °C (lit.)
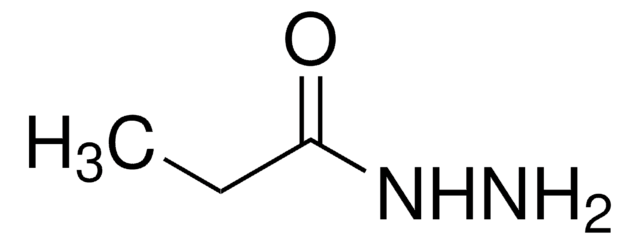
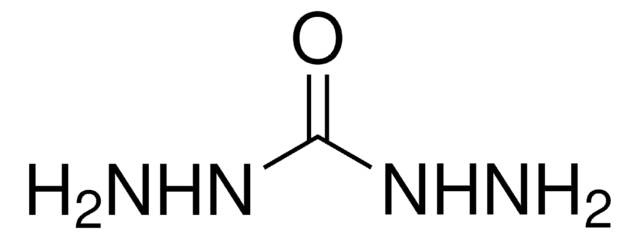
SMILES記法
CC(=O)NNC(C)=O
InChI
1S/C4H8N2O2/c1-3(7)5-6-4(2)8/h1-2H3,(H,5,7)(H,6,8)
InChI Key
ZLHNYIHIHQEHJQ-UHFFFAOYSA-N
シグナルワード
Warning
危険有害性の分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
D8402-5KG:
D8402-BULK:
D8402-VAR:
D8402-25G:
D8402-5G:
この製品を見ている人はこちらもチェック
E B Bhalerao et al.
Indian journal of physiology and pharmacology, 29(2), 83-88 (1985-04-01)
Patients suffering from pulmonary tuberculosis were investigated for the levels of isoniazid (INH) and its metabolites viz. acetyl-INH, mono and diacetyl hydrazines and ammonia. It was observed that 50% of the patients are slow inactivators of INH and almost all
S V Bhide et al.
Cancer letters, 23(2), 235-240 (1984-06-01)
Two hydrazine derivatives, monoacetyl hydrazine (MAH) and diacetyl hydrazine (DAH), have been tested for mutagenic response in the Salmonella/mammalian microsome assay and micronucleus test. MAH but not DAH, increased the revertant mutants in TA100 and TA1535 and also increased the
J A Timbrell et al.
Human toxicology, 3(6), 485-495 (1984-12-01)
The urinary metabolite profile of isoniazid has been studied in patients receiving the drug as therapy for tuberculosis and the profile in patients suffering liver damage due to isoniazid compared with that in control patients. There were no consistent differences
E B Bhalerao et al.
Indian journal of physiology and pharmacology, 29(3), 133-138 (1985-07-01)
Effect of isoniazid (INH) and its metabolites e.g. mono and diacetyl hydrazines (MAH and DAH respectively) was studied on circulating and tissue folates in mice (a species susceptible to INH tumorigenicity) and rats (a species resistant to INH carcinogenicity). It
Ramachandran Azhakar et al.
Dalton transactions (Cambridge, England : 2003), 41(5), 1529-1533 (2011-12-14)
The reaction of N-heterocyclic silylene (NHSi) L [L = CH{(C[double bond, length as m-dash]CH(2))(CMe)(2,6-iPr(2)C(6)H(3)N)(2)}Si] with benzoylhydrazine, 1,2-dicarbethoxyhydrazine, 1,2-diacetylhydrazine and 1,2-bis(tert-butoxycarbonyl)hydrazine in 1 : 1 molar ratio resulted in compounds 1-4 with an almost quantitative yield and five coordinate silicon atoms.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)