おすすめの製品
品質水準
アッセイ
95%
フォーム
powder or crystals
反応適合性
reagent type: cross-linking reagent
官能基
azide
保管温度
2-8°C
SMILES記法
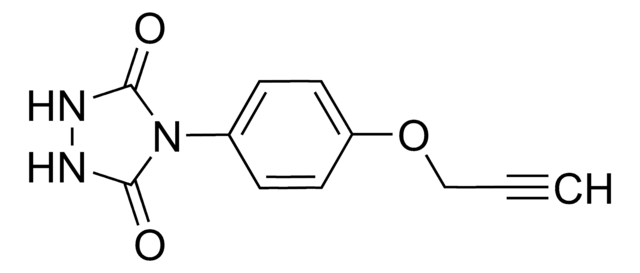
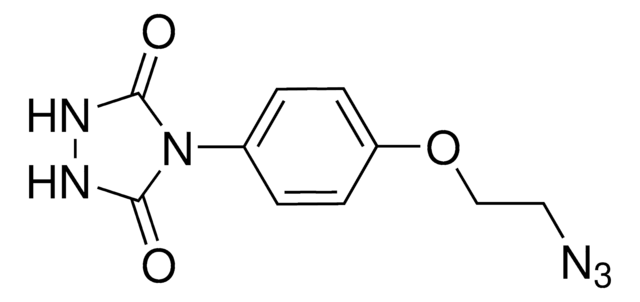
O=C(NNC1=O)N1C2=CC=C(OCCN=[N+]=[N-])C=C2
InChI
1S/C10H10N6O3/c11-15-12-5-6-19-8-3-1-7(2-4-8)16-9(17)13-14-10(16)18/h1-4H,5-6H2,(H,13,17)(H,14,18)
InChI Key
MHGMHPVYCVQIET-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
PTAD-Azide (4-(4-(2-Azidoethoxy)phenyl)-1,2,4-triazolidine-3,5-dione) is a 1,2,4-triazolidine-3,5-dione derivative. It can be prepared from ethyl hydrazinecarboxylate.
アプリケーション
PTAD-Azide is a selective crosslinking reagent that has one end for reacting with tyrosine and the other end for presenting an azide. After bioconjugation to tyrosine, the azide can be reacted with an alkyne through the Cu(I)-catalyzed click chemistry reaction or with a cyclooctyne in a copper-free reaction. This reagent has been shown to selectively introduce poly(ethylene glycol) or PEG chains onto proteins with surface exposed tyrosine residues. PTAD-Azide has also been used in the formation of antibody-drug conjugates. This reagent is compatible with different buffer systems such as PBS, Tris and mixed PBS/Tris buffer (preferred). The linkage with tyrosine has been shown to be stable to pH and temperature extremes as well as blood plasma.
Note: PTAD-Azide must be first activated by stirring in a 1:0.98 molar ratio with 1,3-dibromo-5,5-dimethylhydantoin (product # 157902). Activation is evident upon solution color change from colorless to deep red and the activated reagent should be used immediately.
General Procedure for Protein Modification with PTAD.
Part 1: PTAD activation
Part 2: Protein modification
Note: PTAD-Azide must be first activated by stirring in a 1:0.98 molar ratio with 1,3-dibromo-5,5-dimethylhydantoin (product # 157902). Activation is evident upon solution color change from colorless to deep red and the activated reagent should be used immediately.
General Procedure for Protein Modification with PTAD.
Part 1: PTAD activation
- Mix together 1:0.98 molar equivalents of unactivated PTAD to 1,3-dibromo-5,5-dimethylhydantoin (product # 157902) in organic solvent (preferred solvents are DMF or acetonitrile, avoid using DMSO)
- Color change is observed from colorless/pale yellow to deep red (approximately 5 min of mixing).
- After the solution turns red, store the now activated reagent on ice and use for protein modification within 30 min.
Part 2: Protein modification
- Add protein solution in mixed phosphate/Tris buffer or Tris buffer (pH should be 6 - 9) to the eppendorf tube (or other vial) containing the activated PTAD reagent prepared above and mix gently at room temperature for up to 30 min. Preferably use 10-fold molar excess of reagent relative to protein. Use protein at a minimum concentration of 1 mg/ml (higher concentrations are preferred for enhanced labeling).
- Remove excess unreacted PTAD by gel filtration.
PTAD-Azide may be used in the preparation of 4-(4-(2-azidoethoxy)phenyl)-3H-1,2,4-triazole-3,5(4H)-dione and aplaviroc-urazole. This urazole was recently demonstrated in the traceless, chemoselective labeling of peptides and proteins through electrochemical tyrosine-click (e-Y-CLICK) chemistry.
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
ALD00344-50MG:
ALD00344-VAR:
ALD00344-BULK:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
Hitoshi Ban et al.
Bioconjugate chemistry, 24(4), 520-532 (2013-03-29)
The scope, chemoselectivity, and utility of the click-like tyrosine labeling reaction with 4-phenyl-3H-1,2,4-triazoline-3,5(4H)-diones (PTADs) is reported. To study the utility and chemoselectivity of PTAD derivatives in peptide and protein chemistry, we synthesized PTAD derivatives possessing azide, alkyne, and ketone groups
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)