おすすめの製品
製品名
keYPhos™,
フォーム
powder
品質水準
反応適合性
reagent type: ligand
mp
167-169 °C
官能基
phosphine
SMILES記法
[P](=C(P(C5CCCCC5)C4CCCCC4)C)(C3CCCCC3)(C2CCCCC2)C1CCCCC1
InChI
1S/C32H58P2/c1-27(33(28-17-7-2-8-18-28)29-19-9-3-10-20-29)34(30-21-11-4-12-22-30,31-23-13-5-14-24-31)32-25-15-6-16-26-32/h28-32H,2-26H2,1H3
InChI Key
TZVGFYHCFCNLKS-UHFFFAOYSA-N
詳細
keYPhos™is an ylide-functionalized phosphine ligand developed in the lab of Prof. V. Gessner at the Ruhr-University Bochum with demonstrated uses in Pd-catalyzed cross coupling reactions, including the arylation of ketones and arylation of amines. keYPhos™ is part of the YPhos™ family of ligands, also containing the joYPhos™ and trYPhos™ ligands.
アプリケーション
The electron-rich and sterically demanding keYPhos™ has a methyl group in the ylide-backbone and is a valuable ligand for the palladium catalyzed coupling of aryl chlorides with primary and secondary alkyl and aryl amines at room temperature. keYPhos™has been used in the gold(I)-catalyzed hydroamination of acetylene, and has shown to be effective in a range of Buchwald-Hartwig amination reactions. The strong electron-donor strength and sterically demanding nature of the ligand has been shown to increase the rate of formation of the catalytically active mono-phosphine palladium species, often leading to decreased reaction times or allowing the use of lower reaction temperatures.
Learn more about ylide-functionalized phosphines (YPhos)
Learn more about ylide-functionalized phosphines (YPhos)
特徴および利点
Advantages of the keYPhos™ligand over less electron rich ligand sources include, increased substrate scope in Buchwald-Hartwig amination reactions, including aryl chlorides, the use of more mild reaction conditions and improved activity in in C-N and C-C cross coupling reactions. keYPhos™ has been shown to perform well with common palladium sources such as Pd2(dba)3, Pd(OAc)2, [Pd(allyl)Cl]2 or [Pd(cinamyl)Cl]2.
法的情報
Product of Umicore
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com.
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com.
Yphos is a trademark of Umicore AG & Co. KG
関連製品
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
913294-BULK:
913294-250MG:
913294-1G:
913294-VAR:
最新バージョンのいずれかを選択してください:
Jens Tappen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(19), 4281-4288 (2020-01-24)
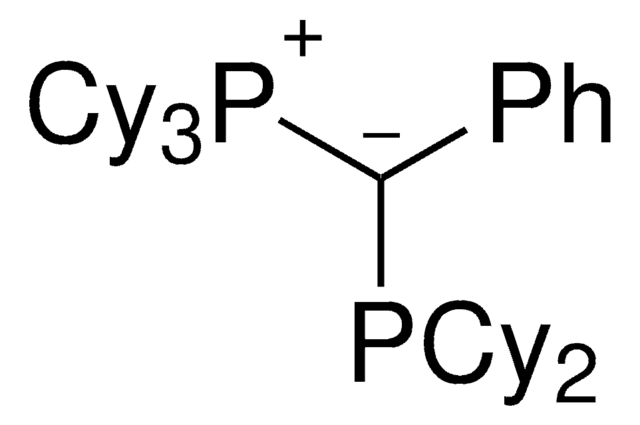
Palladium allyl, cinnamyl, and indenyl complexes with the ylide-substituted phosphines Cy3 P+ -C- (R)PCy2 (with R=Me (L1) or Ph (L2)) and Cy3 P+ -C- (Me)PtBu2 (L3) were prepared and applied as defined precatalysts in C-N coupling reactions. The complexes are
Xiao-Qiang Hu et al.
Organic letters, 21(18), 7558-7562 (2019-08-31)
Ylide-functionalized phosphine (YPhos) ligands allow the palladium-catalyzed α-arylation of alkyl ketones with aryl chlorides with record setting activity. Using a cyclohexyl-substituted YPhos ligand, a wide range of challenging ketone substrates was efficiently and selectively monoarylated under mild conditions. A newly
Ilja Rodstein et al.
The Journal of organic chemistry, 85(22), 14674-14683 (2020-09-11)
Ylide-substituted phosphines have been shown to be excellent ligands for C-N coupling reactions under mild reaction conditions. Here we report studies on the impact of the steric demand of the substituent in the ylide-backbone on the catalytic activity. Two new
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)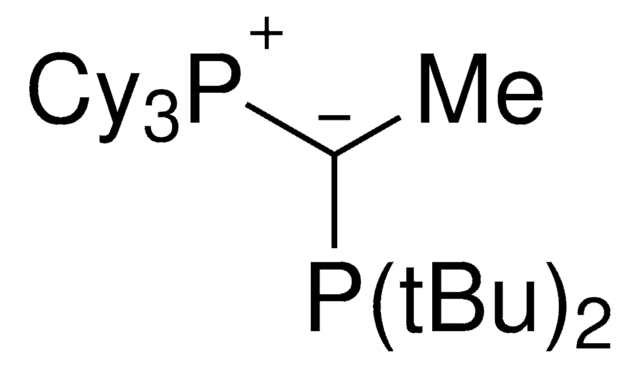
![[1,1′-ビス(ジフェニルホスフィノ)フェロセン]ジクロロパラジウム(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)




![Dichloro[2,2′-bis(diphenylphosphino)-1,1′-binaphthyl]palladium(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/351/904/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3/640/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3.png)