907294
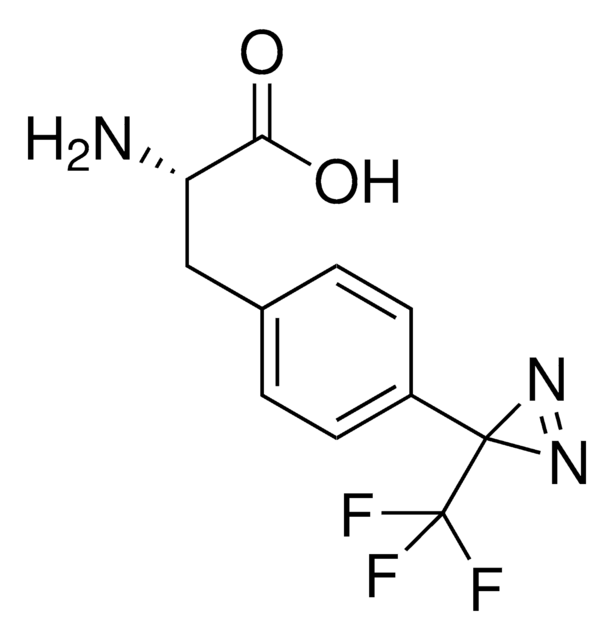
Fmoc-L-Photo-Phe-OH
≥95%
別名:
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)propanoic acid, N-α-(9-Fluorenylmethyloxycarbonyl)-4-(trifluoromethyldiazirin)-L-phenylalanine, Diazirine amino acid, Fmoc-Tdf-OH, Photo-Phe, Photo-crosslinking amino acid, Photoprobe building block
ログイン組織・契約価格を表示する
すべての画像(2)
About This Item
おすすめの製品
アッセイ
≥95%
形状
powder
反応適合性
reaction type: Fmoc solid-phase peptide synthesis
アプリケーション
peptide synthesis
官能基
Fmoc
保管温度
−20°C
アプリケーション
Fmoc-L-Photo-Phe-OH is a diazirine-containing, Fmoc-protected phenylalanine amino acid and multifunctional photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An unprotected version is also available as 907340.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
その他情報
Covalent modifier-type aggregation inhibitor of amyloid-β based on a cyclo-KLVFF motif
Mode of Action of cGMP-dependent Protein Kinase-specific Inhibitors Probed by Photoaffinity Cross-linking Mass Spectrometry
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
Simple and Versatile Method for Tagging Phenyldiazirine Photophores
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Mode of Action of cGMP-dependent Protein Kinase-specific Inhibitors Probed by Photoaffinity Cross-linking Mass Spectrometry
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
Simple and Versatile Method for Tagging Phenyldiazirine Photophores
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
WGK
WGK 3
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
907294-50MG:
907294-VAR:
907294-BULK:
最新バージョンのいずれかを選択してください:
Martijn W H Pinkse et al.
The Journal of biological chemistry, 284(24), 16354-16368 (2009-04-17)
The inhibitor peptide DT-2 (YGRKKRRQRRRPPLRKKKKKH) is the most potent and selective inhibitor of the cGMP-dependent protein kinase (PKG) known today. DT-2 is a construct of a PKG tight binding sequence (W45, LRKKKKKH, KI=0.8 microM) and a membrane translocating sequence (DT-6
Ryuto Kino et al.
Bioorganic & medicinal chemistry letters, 25(15), 2972-2975 (2015-06-06)
Inhibition of amyloid-β (Aβ) aggregation could be a drug development target for treating Alzheimer disease. Insufficient activity to inhibit aggregation, however, remains a key issue. Here, we report a covalent modifier-type aggregation inhibitor of Aβ, diazirine-equipped cyclo-KLVF(β-Ph)F (2). Due to
Michiel A Leeuwenburgh et al.
Organic letters, 8(8), 1705-1708 (2006-04-07)
[reaction: see text] A novel solid-phase synthesis strategy toward succinylhydroxamate peptides, using an appropriately protected hydroxamate building block, is described. Rapid and efficient access is gained to amine-functionalized peptides, which can be decorated with, for instance, a fluorescent label. In
Dany Fillion et al.
Journal of medicinal chemistry, 49(7), 2200-2209 (2006-03-31)
A stereospecific convergent synthesis of N-[(9-fluorenyl)methoxycarbonyl]-p-[3-(trifluoromethyl)-3H-diazirin-3-yl]-l-phenylalanine (Fmoc-12, Fmoc-Tdf) and its incorporation into the C-terminal position of the angiotensin II (AngII) peptide to form (125)I[Sar(1),Tdf(8)]AngII ((125)I-13) is presented. This amino acid photoprobe is a highly reactive carbene-generating diazirine phenylalanine derivative that
David P Smith et al.
Chemical communications (Cambridge, England), (44), 5728-5730 (2008-11-15)
The separative and analytical power of ion mobility spectrometry-mass spectrometry combined with photo-induced cross-linking of site-specifically incorporated trifluoromethyldiazirine provides a powerful approach towards structural characterisation of amyloid fibrils.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)