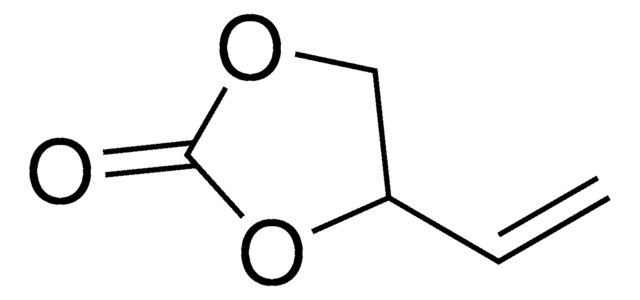
おすすめの製品
品質水準
アッセイ
≥99.0%
フォーム
liquid
環境により配慮した代替製品の特徴
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
屈折率
n20/D 1.445 (lit.)
n20/D 1.447
bp
159.1 °C (lit.)
密度
1.426 g/mL at 25 °C (lit.)
1.433 g/mL at 25 °C
アプリケーション
battery manufacturing
環境により配慮した代替製品カテゴリ
, Enabling
SMILES記法
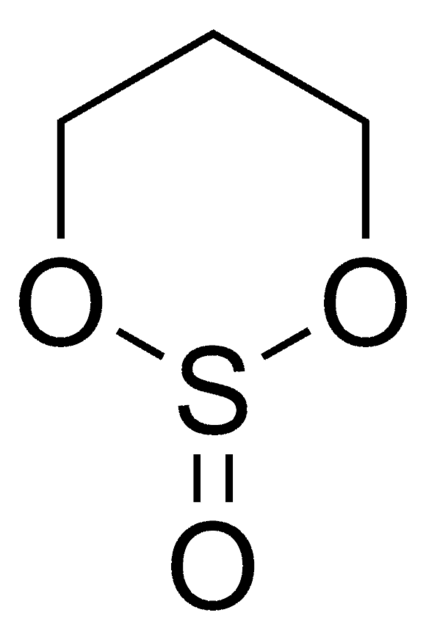
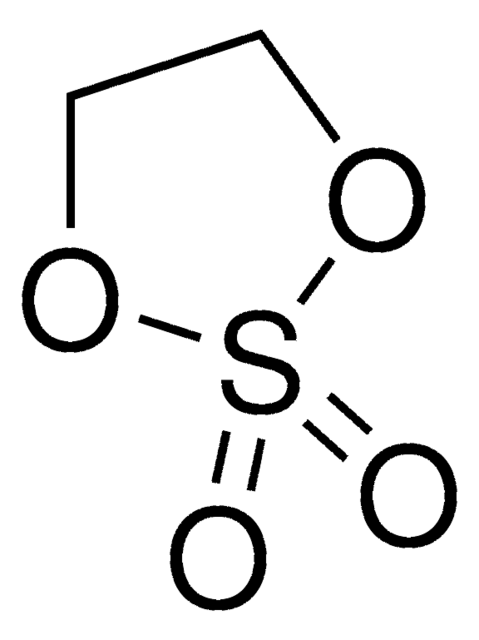
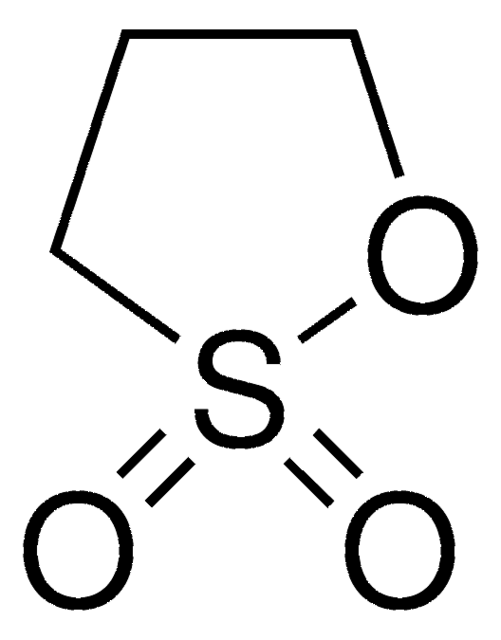
O=S1OCCO1
InChI
1S/C2H4O3S/c3-6-4-1-2-5-6/h1-2H2
InChI Key
WDXYVJKNSMILOQ-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
アプリケーション
関連製品
保管分類コード
10 - Combustible liquids
WGK
WGK 3
引火点(°F)
197.1 °F
引火点(℃)
91.7 °C
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
第三石油類
危険等級III
非水溶性液体
Jan Code
774251-BULK:
774251-25G:
774251-VAR:
この製品を見ている人はこちらもチェック
資料
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
Li-ion batteries are currently the focus of numerous research efforts with applications designed to reduce carbon-based emissions and improve energy storage capabilities.
Li-ion batteries are currently the focus of numerous research efforts with applications designed to reduce carbon-based emissions and improve energy storage capabilities.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
Global Trade Item Number
| カタログ番号 | GTIN |
|---|---|
| 774251-25G | 4061833003015 |
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)