すべての画像(1)
About This Item
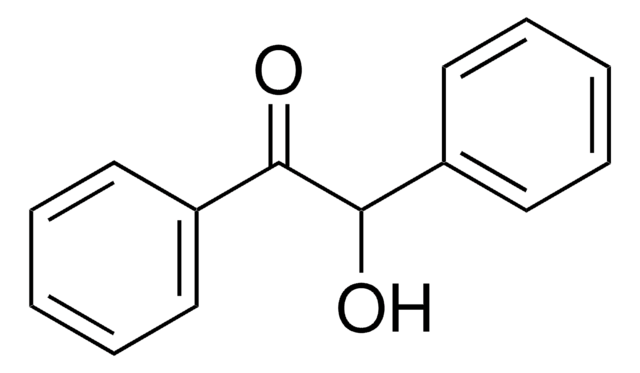
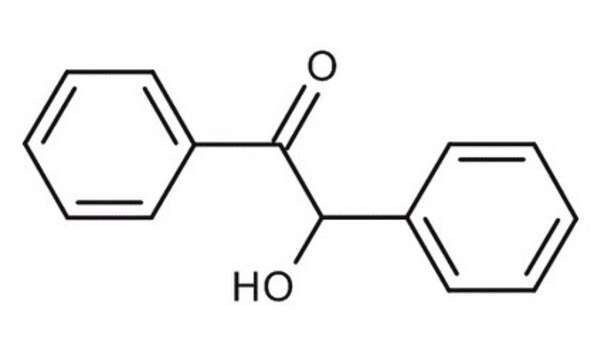
化学式:
C6H5CH(OH)COC6H5
CAS番号:
分子量:
212.24
MDL番号:
UNSPSCコード:
12352115
PubChem Substance ID:
アッセイ:
98%
おすすめの製品
アッセイ
98%
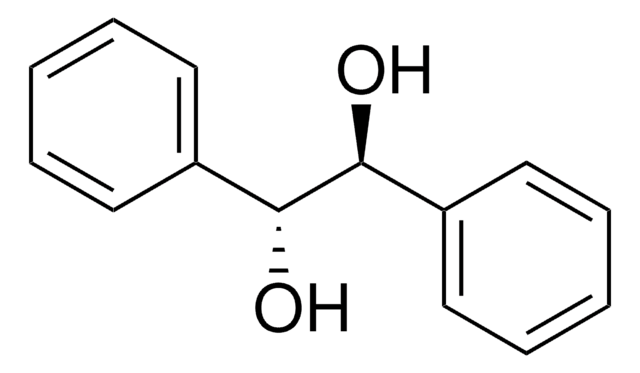
光学活性
[α]24/D −115°, c = 1.5 in acetone
光学純度
ee: 99% (HPLC)
mp
135-137 °C (lit.)
官能基
hydroxyl
ketone
phenyl
SMILES記法
O[C@H](c1ccccc1)C(=O)c2ccccc2
InChI
1S/C14H12O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13,15H/t13-/m1/s1
InChI Key
ISAOCJYIOMOJEB-CYBMUJFWSA-N
アプリケーション
(R)-(-)-Benzoin may be used in the preparation of (R)-2-hydroxy-1-phenylpropanone by reacting with benzaldehyde lyase (BAL) in the presence of acetaldehyde.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
50949:
459941-25MG:
459941-BULK:
459941-VAR:
459941-100MG:
Rüdiger Ohs et al.
Biotechnology progress, 35(6), e2868-e2868 (2019-06-18)
The kinetic description of enzyme-catalyzed reactions is a core task in biotechnology and biochemical engineering. In particular, mechanistic kinetic models help from the discovery of the biocatalyst throughout its application. Chemo- or enantioselective enzyme reactions often undergo two alternative pathways
Hung-Wei Tsui et al.
Journal of chromatography. A, 1637, 461796-461796 (2021-01-03)
The effect of solvents on the enantioselectivities of four structurally similar chiral solutes with a cellulose derivative-based chiral stationary phase, Chiralpak IB, were studied using acetone (AC), 2-propanol (IPA), and tert-butanol (TBA) separately as polar modifiers. The enantioselectivities α of
Rüdiger Ohs et al.
Biotechnology progress, 34(5), 1081-1092 (2018-06-10)
Thiamine diphosphate (ThDP)-dependent enzymes catalyze a broad range of reactions with excellent enantioselectivity. Among these reactions, carboligations of aldehydes are of particular interest since the products, chiral hydroxy ketones, are valuable building blocks in the pharmaceutical industry. However, the substrates
Chromatograms
application for HPLCapplication for HPLCライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)