おすすめの製品
品質水準
アッセイ
98%
形状
solid
bp
219 °C (lit.)
mp
44-47 °C (lit.)
官能基
chloro
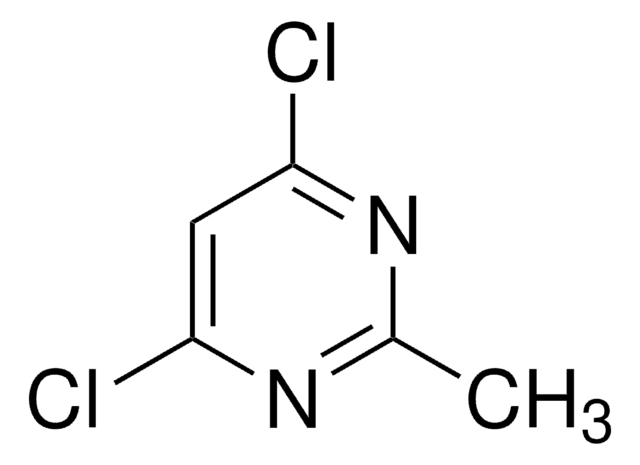
SMILES記法
Cc1cc(Cl)nc(Cl)n1
InChI
1S/C5H4Cl2N2/c1-3-2-4(6)9-5(7)8-3/h2H,1H3
InChI Key
BTLKROSJMNFSQZ-UHFFFAOYSA-N
詳細
2,4-Dichloro-6-methylpyrimidine undergoes double cross-coupling reaction with 2-(tributylstannyl)pyridine, followed by aldol condensation to yield 4-arylvinyl-2,6-di(pyridin-2-yl)pyrimidines. It reacts with 1H,1H,2H,2H-perfluorodecanethiol during fluorous synthesis of disubstituted pyrimidines.
アプリケーション
2,4-Dichloro-6-methylpyrimidine was used in the synthesis of (2-chloro-6-methyl-pyrimidin-4-yl)-(2,3-dihydro-benzothiazol-6-yl)-amine.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Eye Dam. 1 - Skin Corr. 1B
保管分類コード
8A - Combustible corrosive hazardous materials
WGK
WGK 3
引火点(°F)
235.4 °F - closed cup
引火点(℃)
113 °C - closed cup
個人用保護具 (PPE)
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
144185-1KG:
144185-25G:
144185-100G:
144185-VAR:
144185-BULK:
Caroline Hadad et al.
The Journal of organic chemistry, 76(10), 3837-3845 (2011-04-06)
A series of 4-arylvinyl-2,6-di(pyridin-2-yl)pyrimidines have been efficiently prepared by a double cross-coupling reaction between 2,4-dichloro-6-methylpyrimidine and 2-(tributylstannyl)pyridine, followed by aldol condensation with the appropriate aromatic aldehyde substituted with electron-donating, electron-withdrawing, dendritic, or water-soluble groups. The effect of different protic and
Eliud Hernández et al.
Puerto Rico health sciences journal, 29(4), 348-356 (2011-01-26)
Rho family GTPases are molecular switches that control signaling pathways regulating a myriad of cellular functions. Rac1, a Rho family member, plays a critical role in several aspects of tumorigenesis, cancer progression, invasion, and metastasis. Rac proteins are not mutated
Wei Zhang
Organic letters, 5(7), 1011-1013 (2003-03-28)
[reaction: see text] The fluorous synthesis of disubstituted pyrimidines is carried out by attaching 2,4-dichloro-6-methylpyrimidine with 1H,1H,2H,2H-perfluorodecanethiol. The tagged substrate is substituted with 3-(trifluoromethyl)pyrazole followed by thioether oxidation and tag displacement with amines or thiols. The fluorous chain serves as
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)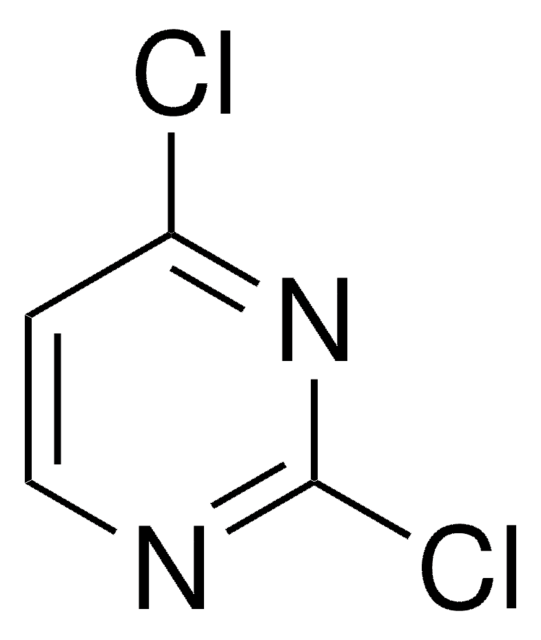

![[1,1′-ビス(ジフェニルホスフィノ)フェロセン]ジクロロパラジウム(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1'-ビス(ジフェニルホスフィノ)フェロセン]ジクロロパラジウム(II)のジクロロメタン複合体](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)