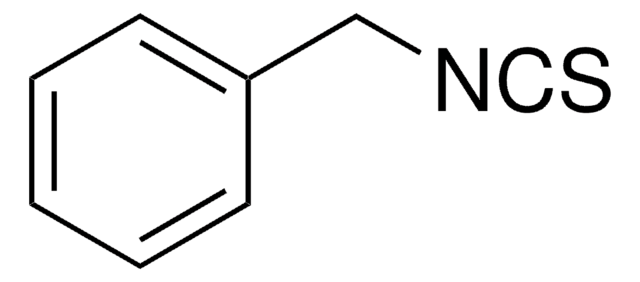
おすすめの製品
品質水準
アッセイ
≥95.0% (GC)
形状
solid
bp
230-235 °C (lit.)
mp
39-41 °C (lit.)
39-41 °C
溶解性
diethyl ether: soluble 0.5 g/10 mL, clear to very faintly turbid, colorless to almost colorless
官能基
phenyl
thiocyanate
thioether
保管温度
2-8°C
SMILES記法
N#CSCc1ccccc1
InChI
1S/C8H7NS/c9-7-10-6-8-4-2-1-3-5-8/h1-5H,6H2
InChI Key
ABNDFSOIUFLJAH-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Benzyl thiocyanate undergoes regioselective bond dissociation during its electrochemical reduction in acetonitrile at an inert electrode. It is added to stimulate the chlortetracycline biosynthesis during industrial fermentations. It undergoes biotransformation into dibenzyl disulphide by Streptomyces aureofaciens.
アプリケーション
Benzyl thiocyanate was used to study the effects of various dietary compounds on the α-hydroxylation of N-nitrosopyrrolidine in male F344 rats in vitro.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3
補足的ハザード
保管分類コード
8A - Combustible corrosive hazardous materials
WGK
WGK 3
引火点(°F)
230.0 °F
引火点(℃)
110 °C
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
13929-VAR:
13929-100G:
13929-BULK:
13929-500G:
S Sugie et al.
Carcinogenesis, 15(8), 1555-1560 (1994-08-01)
The effects of two aromatic thiocyanates, benzyl thiocyanate (BTC) and benzyl isothiocyanate (BITC), on methylazoxymethanol (MAM) acetate-induced intestinal carcinogenesis were examined using female ACI/N rats. Starting at 5 weeks of age, animals were fed diets containing 100 or 400 p.p.m.
F L Chung et al.
Cancer research, 44(7), 2924-2928 (1984-07-01)
Male F344 rats were pretreated with various dietary compounds, and the effects of pretreatment on the in vitro alpha-hydroxylation of N-nitrosopyrrolidine or N'-nitrosonornicotine were determined in assays with liver microsomes or cultured esophagus, respectively. Dietary compounds included phenols, cinnamic acids
Abdelaziz Houmam et al.
Journal of the American Chemical Society, 125(42), 12676-12677 (2003-10-16)
The electrochemical reduction of benzyl thiocyanate and p-nitrobenzyl thiocyanate was investigated in acetonitrile at an inert electrode. These two compounds reveal a change in the reductive cleavage mechanism, and more interestingly, they show a clear-cut example of a regioselective bond
J Fuska et al.
Letters in applied microbiology, 19(3), 124-125 (1994-09-01)
Benzyl thiocyanate, a specific stimulator of chlortetracycline biosynthesis, was transformed into dibenzyl disulphide by Streptomyces aureofaciens. The disulphide stimulated chlortetracycline production to a lesser extent than did benzyl thiocyanate.
X M Li et al.
Applied microbiology and biotechnology, 57(5-6), 717-724 (2002-01-10)
Changes in synthesis and abundance of proteins associated with chlortetracycline (CTC) production in Streptomyces aureofaciens were investigated by two-dimensional polyacrylamide gel electrophoresis of proteins pulse-labelled in vivo with L-[35S]methionine. Eleven individual protein spots were selected as being related to formation
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)