M1650000
Metile 4-idrossibenzoato
European Pharmacopoeia (EP) Reference Standard
Sinonimo/i:
4-idrossibenzoato di metile, Metil estere dell’acido p-idrossibenzoico, Metilparaben
About This Item
Grado
pharmaceutical primary standard
Famiglia di API
parabens
Produttore/marchio commerciale
EDQM
Punto di fusione
125-128 °C (lit.)
applicazioni
pharmaceutical (small molecule)
Formato
neat
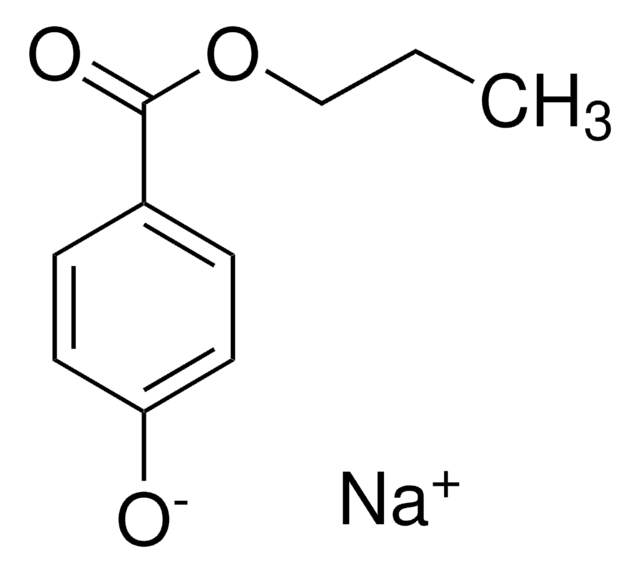
Stringa SMILE
COC(=O)c1ccc(O)cc1
InChI
1S/C8H8O3/c1-11-8(10)6-2-4-7(9)5-3-6/h2-5,9H,1H3
LXCFILQKKLGQFO-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
For further information and support please go to the website of the issuing Pharmacopoeia.
Applicazioni
Established for use according to European Pharmacopoeia for the preparation of the below-given solutions:
- Reference solutions (a) and (b) in the identification, testing for related substances, and assay of methyl parahydroxybenzoate and sodium methyl parahydroxybenzoate, according to the monographs 0409 and 1262
- Reference solution (c) for the testing of related substances in nifuroxazide using liquid chromatography (2.2.29), according to the monograph 1999
- Reference solution (b) in the identification of sodium ethyl parahydroxybenzoate using thin-layer chromatography (General text 2.2.27), according to the monograph 2134
Confezionamento
Altre note
Prodotti correlati
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 2
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
334.4 °F
Punto d’infiammabilità (°C)
168 °C
Choose from one of the most recent versions:
Certificati d'analisi (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.