158534
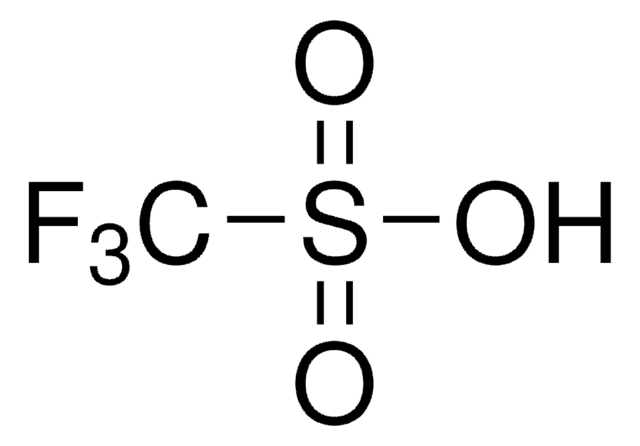
Trifluoromethanesulfonic acid
reagent grade, 98%
Sinonimo/i:
TFMSA, Triflic acid
About This Item
Prodotti consigliati
Grado
reagent grade
Livello qualitativo
Densità del vapore
5.2 (vs air)
Tensione di vapore
8 mmHg ( 25 °C)
Saggio
98%
Forma fisica
liquid
Indice di rifrazione
n20/D 1.327 (lit.)
P. eboll.
162 °C (lit.)
Densità
1.696 g/mL at 25 °C (lit.)
Gruppo funzionale
fluoro
triflate
Stringa SMILE
OS(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S/c2-1(3,4)8(5,6)7/h(H,5,6,7)
ITMCEJHCFYSIIV-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Friedel-Crafts acylation of aromatic compounds with methyl benzoate.
- Addition reaction of dialkyl disulfides to terminal alkynes.
- Synthesis of a single cyclic tetrasiloxane containing propylammonium trifluoromethanesulfonate and methyl side-chain groups (Am-CyTS).
- Preparation of starting reagents for the synthesis of fluorinated 2,5-substituted 1-ethyl-1H-benzimidazole derivatives.
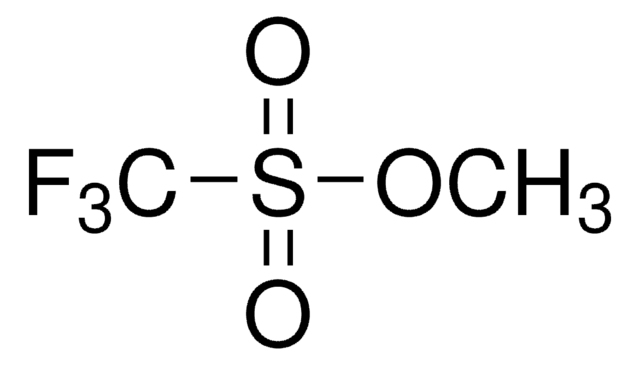
- Synthesis of aryl triflates, the lactonization of alkenoic acids, and the formation of E-alkenes.
Accessorio
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Met. Corr. 1 - Skin Corr. 1B - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
>332.1 °F - Pensky-Martens closed cup
Punto d’infiammabilità (°C)
> 166.7 °C - Pensky-Martens closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
Protocolli
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.