W241512
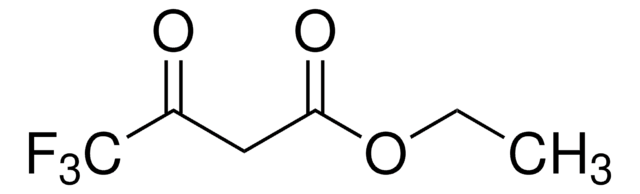
Ethyl acetoacetate
natural, ≥97%, FG
Sinonimo/i:
Acetoacetic ester
About This Item
Prodotti consigliati
Grado
FG
Fragrance grade
Halal
Kosher
natural
agenzia
follows IFRA guidelines
Conformità normativa
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
Densità del vapore
4.48 (vs air)
Tensione di vapore
1 mmHg ( 28.5 °C)
Saggio
≥97%
Temp. autoaccensione
580 °F
Limite di esplosione
9.5 %
Caratteristiche più verdi
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Indice di rifrazione
n20/D 1.418-1.421
P. ebollizione
181 °C (lit.)
Punto di fusione
−43 °C (lit.)
Solubilità
water: soluble 35 part
organic solvents: soluble
Densità
1.029 g/mL at 20 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Allergene in fragranze
no known allergens
Categoria alternativa più verde
Organolettico
apple; fatty; green; fruity
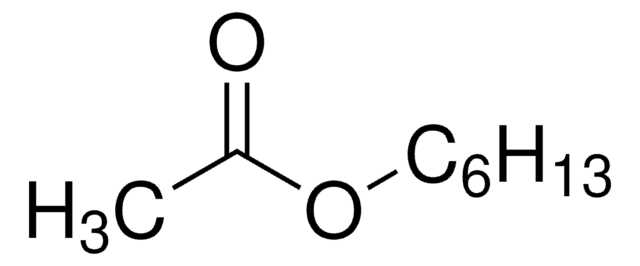
Stringa SMILE
CCOC(=O)CC(C)=O
InChI
1S/C6H10O3/c1-3-9-6(8)4-5(2)7/h3-4H2,1-2H3
XYIBRDXRRQCHLP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Benchtop (19)F Nuclear Magnetic Resonance (NMR) Spectroscopy Provides Mechanistic Insight into the Biginelli Condensation toward the Chemical Synthesis of Novel Trifluorinated Dihydro- and Tetrahydropyrimidinones as Antiproliferative Agents.: This study explores the use of Ethyl acetoacetate in the synthesis of novel trifluorinated compounds with potential antiproliferative properties against cancer cells. The research employs advanced NMR spectroscopy to elucidate the reaction mechanism (Chen et al., 2023, Chen et al., 2023).
- Synthesis and Characterization of New Dihydronaphthalene Candidates as Potent Cytotoxic Agents against MCF-7 Human Cancer Cells.: This article discusses the creation of dihydronaphthalene derivatives using Ethyl acetoacetate, highlighting their significant cytotoxic activity against breast cancer cells. The synthesized compounds show promise for further development as chemotherapeutic agents (Ahmed et al., 2020, Ahmed et al., 2020).
- Synthesis and characterization of new 4H-chromene-3-carboxylates ensuring potent elastase inhibition activity along with their molecular docking and chemoinformatics properties.: Utilizing Ethyl acetoacetate, this research focuses on developing 4H-chromene derivatives that exhibit strong elastase inhibition, a key enzyme implicated in various inflammatory diseases. The study integrates molecular docking and chemoinformatics for detailed analysis (Dige et al., 2020, Dige et al., 2020).
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
164.3 °F - closed cup
Punto d’infiammabilità (°C)
73.5 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W241512-100G-K | 4061834404071 |
| W241512-1KG-K | 4061837876431 |
| W241512-5KG | |
| W241512-100G | |
| W241512-1DRUM-K | |
| W241512-1KG | |
| W241512-5KG-K | 4061837512285 |
| W241512-SAMPLE | |
| W241512-SAMPLE-K | 4061837876448 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.