791571
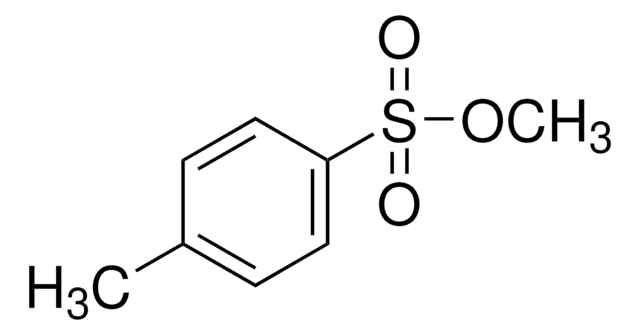
Isopropyl p-toluenesulfonate
97%
Sinonimo/i:
Isopropyl p-tosylate
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H14O3S
Numero CAS:
Peso molecolare:
214.28
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Stato
liquid
Indice di rifrazione
n20/D 1.503
Densità
1.142 g/mL at 25 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
[S](=O)(=O)(Oc1ccc(cc1)C)C(C)C
InChI
1S/C10H14O3S/c1-8(2)14(11,12)13-10-6-4-9(3)5-7-10/h4-8H,1-3H3
MUTOLSSCMLECRU-UHFFFAOYSA-N
Applicazioni
Isopropyl p-toluenesulfonate can be used as a reactant:
- For the alkylation of amines in the presence of triethylamine.
- For the O-alkylation of cyclic thiohydroxamic acids in the presence of tetrabutylammonium hydroxide.
- To synthesize 3-methyl-2-phenyl-1-butanol by reacting with styrene in the presence of bromo(2-cyclohexylethyl)magnesium and zirconocene dichloride.
Isopropyl p-toluenesulfonate can be used:
- as a thermal acid generator that synthesizes inherently stretchable p-conjugated polymer
- as a catalyst for the deprotection of PBHEMA [Poly(2-(tert-butoxycarbonyloxy)ethyl methacrylate)] on heating
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Design, Synthesis and Antimicrobial Evaluation of Novel Tricyclic Benzoxazine Fluoroquinolones under Conventional and Microwave Methods
Guruswamy B, et al.
Journal of Heterocyclic Chemistry, 52, 532-538 (2015)
B Y P Tay et al.
International journal of cosmetic science, 38(6), 627-633 (2016-05-14)
Isopropyl p-toluenesulfonate (IPTS) is a potentially genotoxic by-product formed during the esterification of palm oil-based palmitic and palm kernel oil-based myristic acid with isopropanol to produce isopropyl palmitate or isopropyl myristate. There are no methods described for the analysis of
J de Armas et al.
Organic letters, 3(13), 2097-2100 (2001-06-22)
[reaction: see structure] The first examples of efficient electrophilic Zr-catalyzed carbomagnesations are disclosed, where in contrast to previous catalytic carbomagnesations the alkyl moiety of the electrophile is transferred (vs that of the Grignard reagent). The identity of the Grignard reagent
Satsuki Chikura et al.
Mutation research, 811, 110-116 (2016-12-10)
As part of a collaborative study in the Mammalian Mutagenicity Study group of the Japanese Environmental Mutagen Society, we evaluated the in vivo mutagenicity of isopropyl p-toluenesulfonate (IPTS) using a peripheral blood Pig-a assay in rats. Pig-a mutant frequency (MF)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.