722081
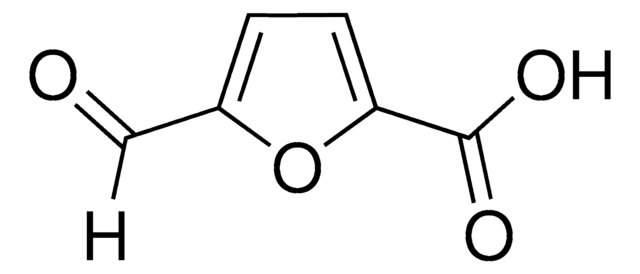
2,5-Furandicarboxylic acid
97%
Sinonimo/i:
Dehydromucic acid, FDCA
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H4O5
Numero CAS:
Peso molecolare:
156.09
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Stato
powder
Punto di fusione
>300 °C
Gruppo funzionale
carboxylic acid
Stringa SMILE
OC(=O)c1ccc(o1)C(O)=O
InChI
1S/C6H4O5/c7-5(8)3-1-2-4(11-3)6(9)10/h1-2H,(H,7,8)(H,9,10)
CHTHALBTIRVDBM-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
2,5-Furandicarboxylic acid (FDCA) is a renewable, greener substitute for terephthalate in the production of polyesters. It is widely used as a precursor for the synthesis of bio-based polyesters and various other polymers.
Applications of FDCA in the synthesis of several metal-organic frameworks (MOFs) have also been reported.
Applications of FDCA in the synthesis of several metal-organic frameworks (MOFs) have also been reported.
Altre note
2,5-Furandicarboxylic acid has been included among Top 10 biorefinery carbohydrate derivatives for the production of biobased industrial products.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
The furan counterpart of poly (ethylene terephthalate): An alternative material based on renewable resources.
Gandini A, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 47(1), 295-298 (2009)
Polyesters derived from furan and tetrahydrofuran nuclei.
Moore J A and Kelly J E
Macromolecules, 11(3), 568-573 (1978)
Crystalline Capsules: Metal?Organic Frameworks Locked by Size?Matching Ligand Bolts.
Wang H, et al.
Angewandte Chemie (International Edition in English), 54(20), 5966-5970 (2015)
Giulia Guidotti et al.
International journal of molecular sciences, 20(9) (2019-05-06)
Biopolymers are gaining increasing importance as substitutes for plastics derived from fossil fuels, especially for packaging applications. In particular, furanoate-based polyesters appear as the most credible alternative due to their intriguing physic/mechanical and gas barrier properties. In this study, block
Niki Poulopoulou et al.
Polymers, 12(1) (2020-01-23)
Intending to expand the thermo-physical properties of bio-based polymers, furan-based thermoplastic polyesters were synthesized following the melt polycondensation method. The resulting polymers, namely, poly(ethylene 2,5-furandicarboxylate) (PEF), poly(propylene 2,5-furandicarboxylate) (PPF), poly(butylene 2,5-furandicarboxylate) (PBF) and poly(1,4-cyclohexanedimethylene 2,5-furandicarboxylate) (PCHDMF) are used in blends
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.