579920
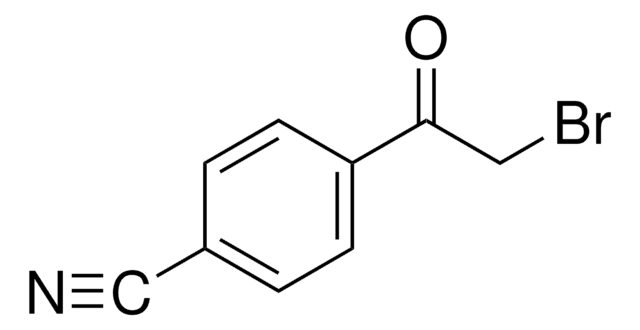
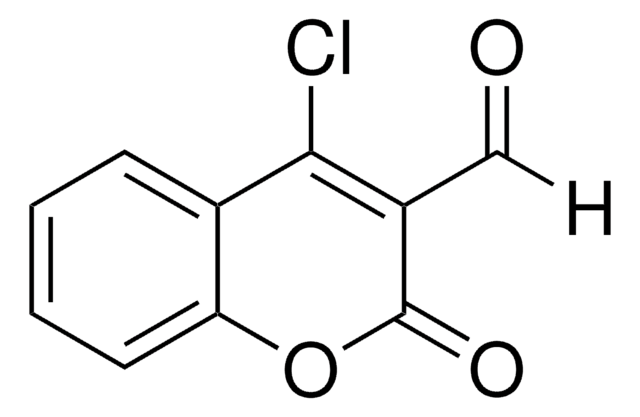
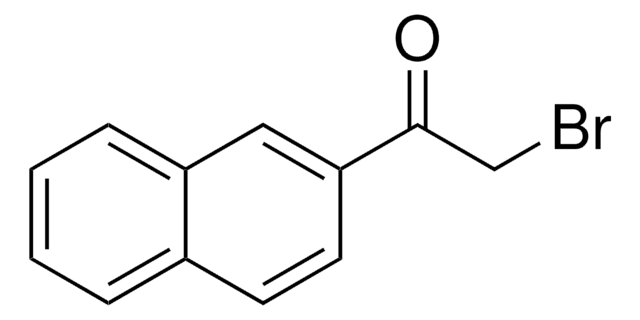
3-(Bromoacetyl)coumarin
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C11H7BrO3
Numero CAS:
Peso molecolare:
267.08
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Forma fisica
solid
Punto di fusione
164-168 °C (lit.)
Stringa SMILE
BrCC(=O)C1=Cc2ccccc2OC1=O
InChI
1S/C11H7BrO3/c12-6-9(13)8-5-7-3-1-2-4-10(7)15-11(8)14/h1-5H,6H2
NTYOLVNSXVYRTJ-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
3-(Bromoacetyl)coumarin can be synthesized via the bromination of 3-acetylcoumarin in chloroform.
Applicazioni
3-(bromoacetyl)coumarin may be used in the synthesis of the following:
- 3-(bromoacetyl)coumarin oxime via reaction with hydroxylamine hydrochloride in methanol
- 3-(bromoacetyl)coumarin-O-methyloxime via reaction with O-benzylhydroxylammonium chloride/diluted HCl in methanol
- 3-(bromoacetyl)coumarin-O-benzyloxime via reaction with O-benzyl hydroxylamine hydrochloride in methanol
- 3-[2′-(2′′-arylidenehydrazinyl)thiazolyl]coumarins via reaction with benzaldehyde thiosemicarbazones
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
"Synthesis and Antibacterial Activity of Quinolone-Based Compounds Containing a Coumarin Moiety"
Emami S, et al.
Arch. Pharm. (Weinheim), 341(01), 42-48 (2008)
"Synthesis and Oral Hypoglycemic Activity of 3-[5'-Methyl-2'-aryl-3'-(thiazol-2"-yl amino) thiazolidin-4'-one] coumarin Derivatives"
Kini D and Ghate M
Journal of Chemistry, 8(01), 386- 390 (2011)
Saman Khan et al.
Chemico-biological interactions, 290, 64-76 (2018-05-29)
Coumarin is an important bioactive pharmacophore. It is found in plants as a secondary metabolite and exhibits diverse pharmacological properties including anticancer effects against different malignancies. Therapeutic efficacy of coumarin derivatives depends on the pattern of substitution and conjugation with
Ewa Poboży et al.
Mikrochimica acta, 172(3-4), 409-417 (2011-04-08)
Perfluorinated carboxylic acids (PFCAs) represent an important group of persistent perfluorinated organic compounds commonly determined in environmental and biological samples. A reversed-phase HPLC method was developed based on derivatization of the PFCAs with the commercially available fluorescent reagent 3-bromoacetyl coumarin.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.