520446
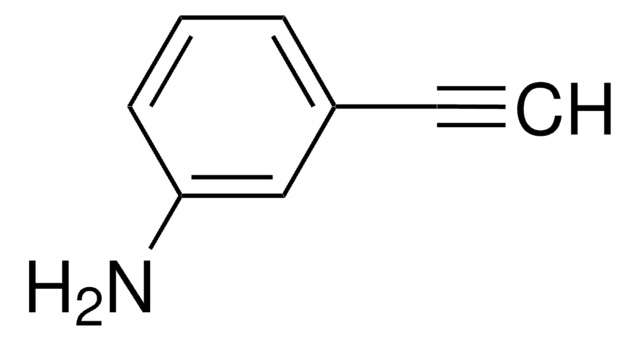
3-Ethynylpyridine
98%
About This Item
Prodotti consigliati
Saggio
98%
P. eboll.
83-84 °C/30 mmHg (lit.)
Punto di fusione
39-40 °C (lit.)
Stringa SMILE
C#Cc1cccnc1
InChI
1S/C7H5N/c1-2-7-4-3-5-8-6-7/h1,3-6H
CLRPXACRDTXENY-UHFFFAOYSA-N
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
4.1B - Flammable solid hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
134.0 °F - closed cup
Punto d’infiammabilità (°C)
56.67 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
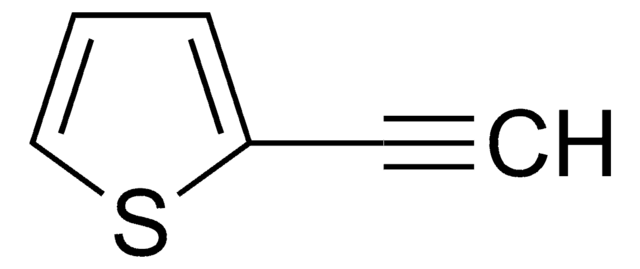
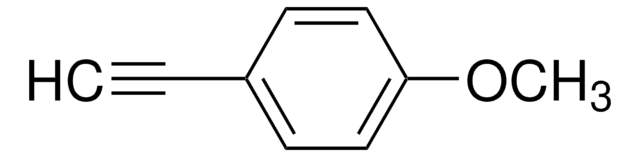
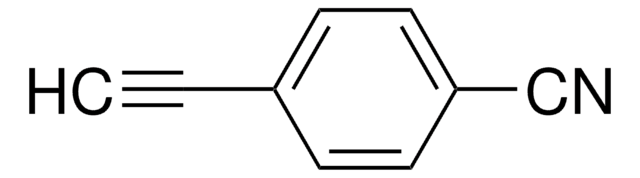
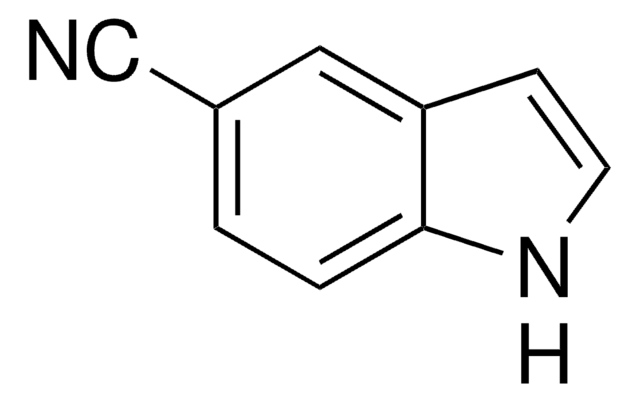
I clienti hanno visto anche
Articoli
The terminal alkyne functionality has a wide range of applications including most recently the synthesis of spiropyran substituted 2,3-dicyanopyrazines and (±)-asteriscanolide, as well as conversion to enamines using resin-bound 2° amines.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.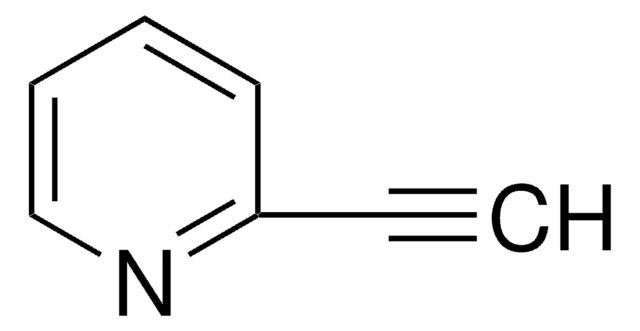



![3-[(Trimethylsilyl)ethynyl]pyridine 97%](/deepweb/assets/sigmaaldrich/product/structures/343/531/3049f5ac-7c3c-45ca-b43c-809abc2f3c9d/640/3049f5ac-7c3c-45ca-b43c-809abc2f3c9d.png)