448109
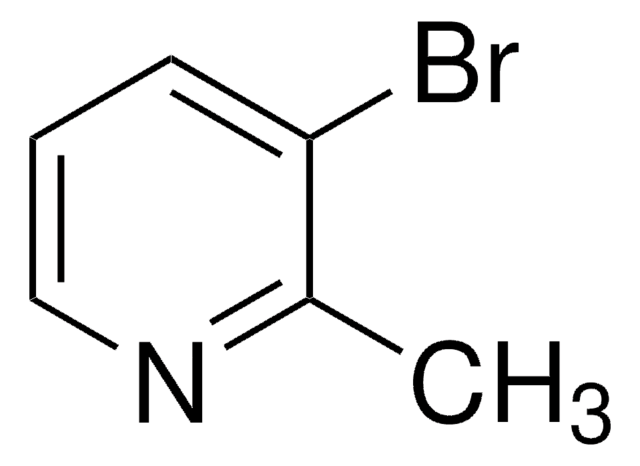
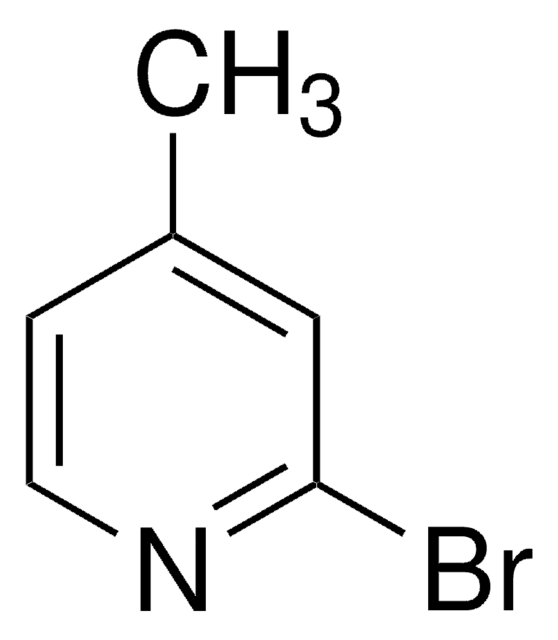
2-Bromo-3-methylpyridine
95%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H6BrN
Numero CAS:
Peso molecolare:
172.02
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
95%
Indice di rifrazione
n20/D 1.568 (lit.)
P. ebollizione
218-219 °C (lit.)
Densità
1.544 g/mL at 25 °C (lit.)
Gruppo funzionale
bromo
Stringa SMILE
Cc1cccnc1Br
InChI
1S/C6H6BrN/c1-5-3-2-4-8-6(5)7/h2-4H,1H3
PZSISEFPCYMBDL-UHFFFAOYSA-N
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Kelnner W R de França et al.
The Journal of organic chemistry, 67(6), 1838-1842 (2002-03-16)
2,2'-Bipyridine (bpy) and a series of dimethyl-2,2'-bipyridines were synthesized from 2-bromopyridine and 2-bromomethylpyridines, respectively, using an electrochemical process catalyzed by nickel complexes. The method is simple and efficient, with isolated yields between 58 and 98% according to the structure. We
Lynne H Thomas et al.
Acta crystallographica. Section C, Crystal structure communications, 69(Pt 11), 1279-1288 (2013-11-07)
Controlled introduction of proton transfer into the design of a series of molecular complexes is described, delivering the systematic production of ionic molecular complexes (molecular salts). The controlled production of molecular salts has relevance as a potential strategy in the
Synthesis of 2, 2'-Bipyridines: Versatile Building Blocks for Sexy Architectures and Functional Nanomaterials.
European Journal of Organic Chemistry, 2, 235-254 (2004)
Copper (ii) complexes of 2-halo-3-methylpyridine: synthesis, structure, and magnetic behaviour of Cu (2-X-3-CH 3 py) 2 X' 2 [X, X'= chlorine or bromine; py= pyridine].
Herringer SN, et al.
Dalton Transactions, 40(16), 4242-4252 (2011)
Alan C Spivey et al.
Organic letters, 9(5), 891-894 (2007-02-10)
[reaction: see text] The scope and limitations of the conjugate addition of 2- and the first 4-pyridyl Gilman homocuprates to various alpha,beta-unsaturated Michael acceptors are delineated. The conjugate addition of the cuprate of 2-bromo-3-methylpyridine to (E)-methyl crotonate then diastereoselective enolate
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.