419648
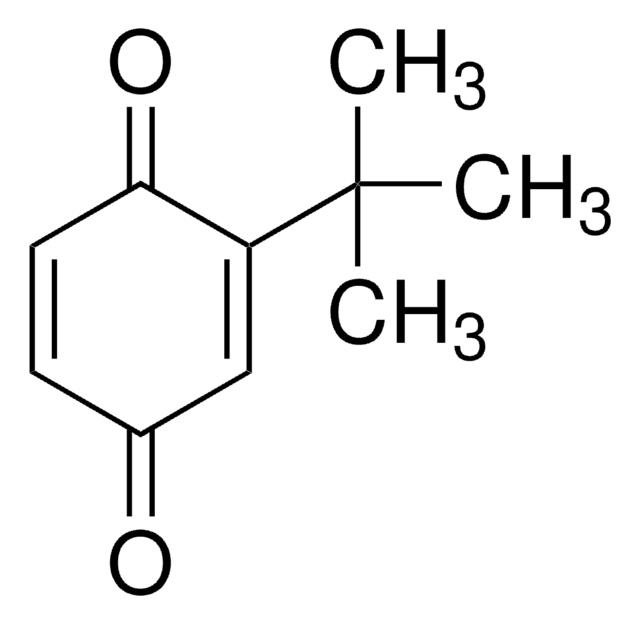
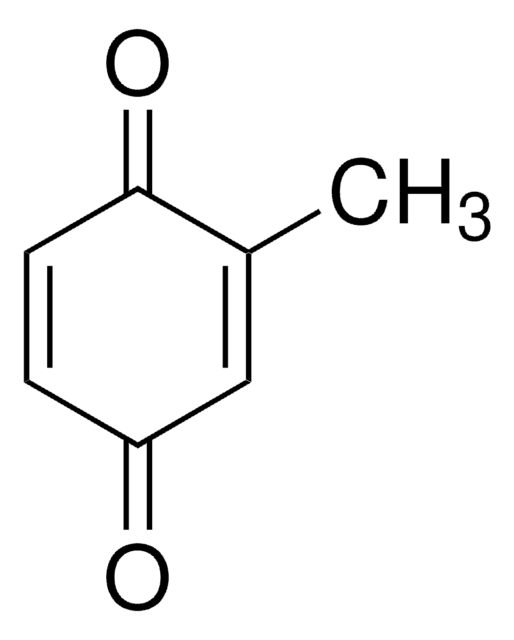
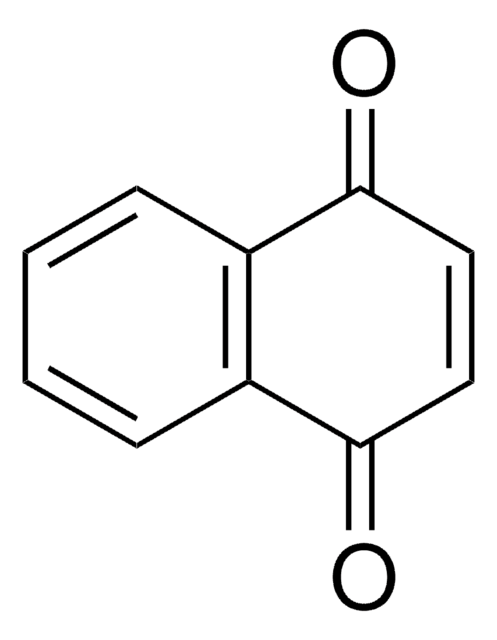
2,5-Di-tert-butyl-1,4-benzoquinone
99%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
[(CH3)3C]2C6H2(=O)2
Numero CAS:
Peso molecolare:
220.31
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Punto di fusione
152-154 °C (lit.)
Gruppo funzionale
ketone
Stringa SMILE
CC(C)(C)C1=CC(=O)C(=CC1=O)C(C)(C)C
InChI
1S/C14H20O2/c1-13(2,3)9-7-12(16)10(8-11(9)15)14(4,5)6/h7-8H,1-6H3
ZZYASVWWDLJXIM-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
2,5-Di-tert-butyl-1,4-benzoquinone (DTBBQ) is an 2,5-disubstituted quinone. It is an antibacterial compound. It has been isolated from marine Streptomyces sp. VITVSK1. Pressure dependance on the intramolecular and intermolecular migration rates of Na+ and K+ in a 2,5-di-tert-butyl-1,4-benzoquinone ion pair have been evaluated by using a high-pressure EPR technique.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Catalytic aerobic deamination of activated primary amines by a model for the quinone cofactor of mammalian copper amine oxidases.
L M Sayre et al.
Methods in enzymology, 258, 53-69 (1995-01-01)
L C Rome et al.
The Journal of physiology, 526 Pt 2, 279-286 (2000-07-15)
1. The rate at which an isometrically contracting muscle uses energy is thought to be proportional to its twitch speed. In both slow and fast muscles, however, a constant proportion (25-40 %) of the total energy has been found to
Vinay Gopal Jannu et al.
International journal of bioinformatics research and applications, 11(2), 142-152 (2015-03-20)
The incidence of bacterial disease has increased tremendously in the last decade, because of the emergence of drug resistance strains within the bacterial pathogens. The present study was to investigate the antibacterial compound 2,5-di-tert-butyl-1,4-benzoquinone (DTBBQ) isolated from marine Streptomyces sp.
L Missiaen et al.
European journal of pharmacology, 227(4), 391-394 (1992-12-01)
Specific inhibitors of the endoplasmic-reticulum Ca2+ pump will deplete intracellular stores and are therefore useful to study the role of store depletion on plasma-membrane Ca2+ permeability. We now report that the Ca(2+)-pump inhibitor 2,5-di-(tert-butyl)-1,4-benzohydroquinone (tBuBHQ) reduces the passive Ca2+ leak
R J Dolor et al.
The American journal of physiology, 262(1 Pt 1), C171-C181 (1992-01-01)
We have investigated the role of the intracellular Ca2+ pool in regulating Ca2+ entry into vascular endothelial cells. The intracellular Ca2+ pool was mobilized using either thapsigargin (TG) or 2',5'-di(tert-butyl)-1,4-benzohydroquinone (BHQ), inhibitors of the endoplasmic reticulum Ca(2+)-adenosinetriphosphatase (ATPase). Mobilization of
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 419648-25G | |
| 419648-5G | 4061831820256 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.