428566
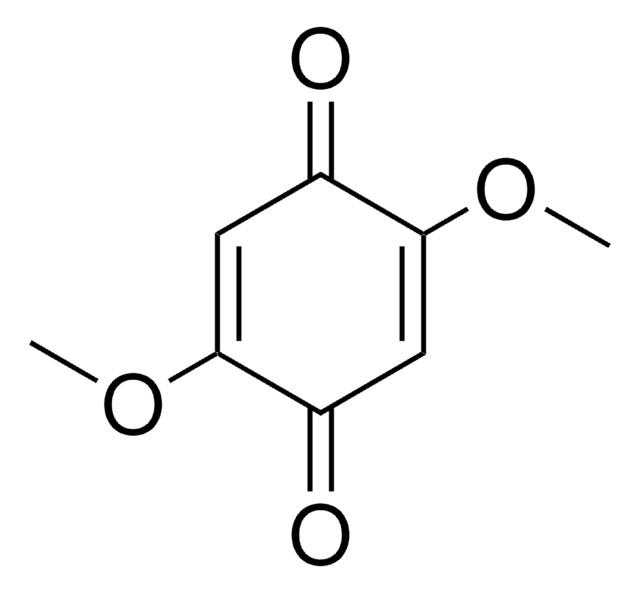
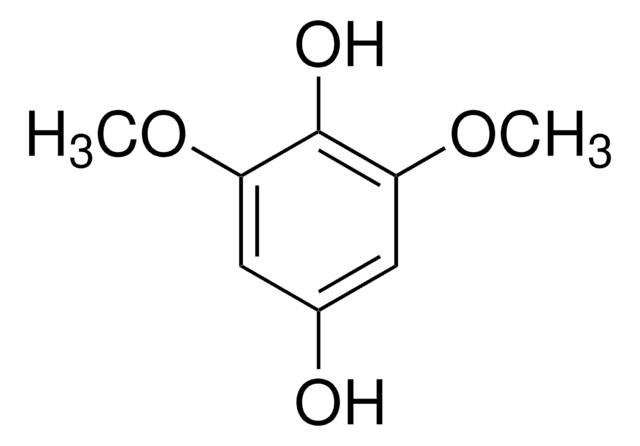
2,6-Dimethoxy-1,4-benzoquinone
97%
Sinonimo/i:
DMBQ
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C8H8O4
Numero CAS:
Peso molecolare:
168.15
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Punto di fusione
253-257 °C (dec.) (lit.)
Solubilità
DMSO: soluble(lit.)
Gruppo funzionale
ether
ketone
Stringa SMILE
COC1=CC(=O)C=C(OC)C1=O
InChI
1S/C8H8O4/c1-11-6-3-5(9)4-7(12-2)8(6)10/h3-4H,1-2H3
OLBNOBQOQZRLMP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
2,6-Dimethoxy-1,4-benzoquinone (DBQ, 2,6-DMBQ, DMOBQ) is a 1,4-benzoquinone derivative. It is a wood allergen, has been reported to cause various skin and mucosal symptoms on exposure to wood dusts. It is formed as a product due to the activity of bacterial Azospirillum lipoferum laccase on phenolic compounds of the syringic type. DBQ is one of the components isolated from the rhizome of Gynura japonica with a potential to show anti-platelet aggregation activity in vitro. It is an anticancer agent, whose kinetics of cyclic redox transformation induced by ascorbate (AscH-) has been studied using the Clark electrode and ESR techniques. Its electrochemical reduction in acetonitrile has been studied.
Applicazioni
2,6-Dimethoxy-1,4-benzoquinone may be used in the synthesis of 2-aryl-3,5-dimethoxy-1,4-benzoquinone derivatives.
Known haustorial inducing factor.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Fang-Rong Chang et al.
Journal of natural products, 65(3), 255-258 (2002-03-23)
Three new eudesmanolide sesquiterpenes, neolitacumone A-C (1-3), and one new benzylisoquinoline alkaloid, neolitacumonine (5), along with 27 known compounds were isolated from the stem bark of Neolitsea acuminatissima. The structures of compounds 1-3 and 5 were established on the basis
Lelde Krumina et al.
Environmental science & technology, 51(16), 9053-9061 (2017-07-12)
Hydroquinones are important mediators of electron transfer reactions in soils with a capability to reduce Fe(III) minerals and molecular oxygen, and thereby generating Fenton chemistry reagents. This study focused on 2,6-dimethoxy hydroquinone (2,6-DMHQ), an analogue to a common fungal metabolite
V A Roginsky et al.
Biochemistry. Biokhimiia, 63(2), 200-206 (1998-06-02)
The kinetics of cyclic redox transformation of 2,6-dimethoxy-1, 4-benzoquinone (DMOBQ)--the well-known effective anticancer agent--induced by ascorbate (AscH-) were studied in phosphate buffer, pH 7.40, at 37 degreesC using the Clark electrode and ESR techniques. The process is due to the
Anomalous behavior in the two-step reduction of quinones in acetonitrile.
Lehmann MW and Evans DH.
Journal of Electroanalytical Chemistry, 500(1), 12-20 (2001)
Quy A Ngo et al.
BMC plant biology, 13, 28-28 (2013-02-20)
Plant parasitism represents an extraordinary interaction among flowering plants: parasitic plants use a specialized organ, the haustorium, to invade the host vascular system to deprive host plants of water and nutrients. Various compounds present in exudates of host plants trigger
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.