357987
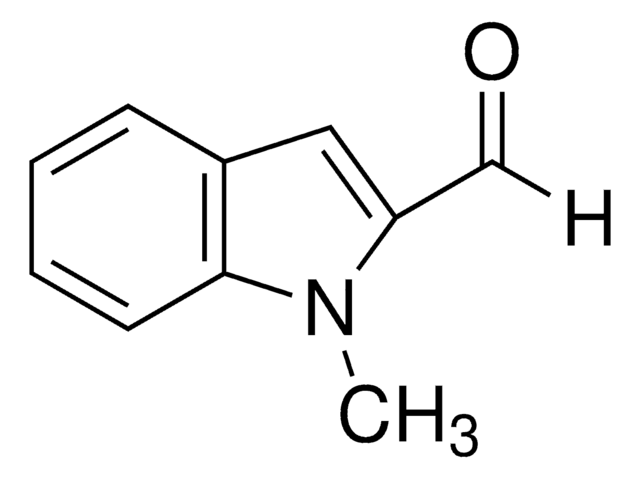
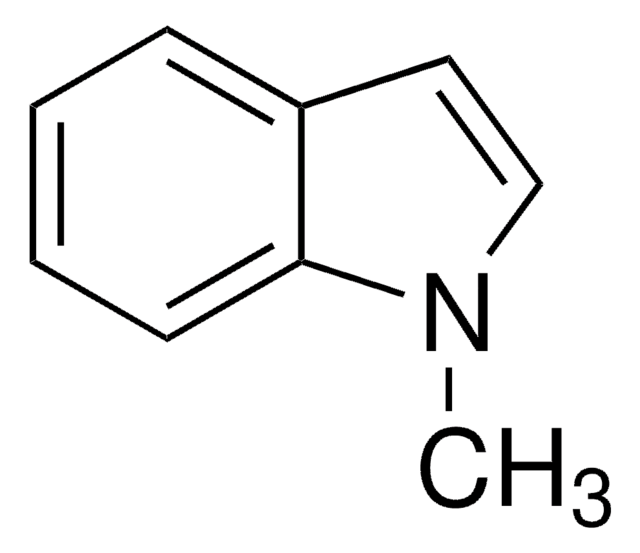
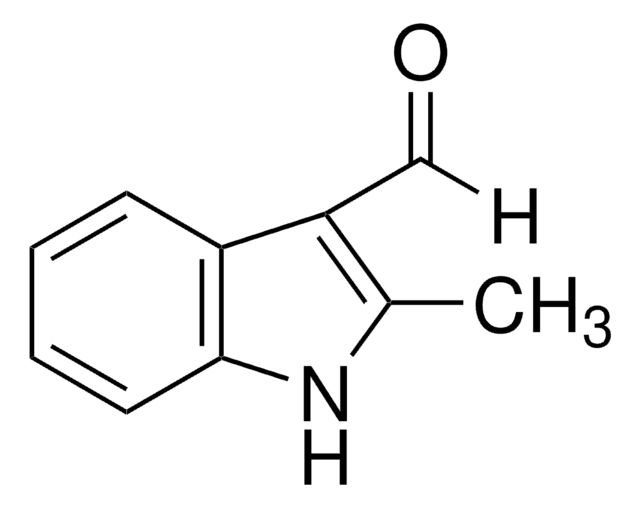
1-Methylindole-3-carboxaldehyde
97%
Sinonimo/i:
3-Formyl-1-methylindole, NSC 83042
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H9NO
Numero CAS:
Peso molecolare:
159.18
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Punto di fusione
70-72 °C (lit.)
Gruppo funzionale
aldehyde
Stringa SMILE
Cn1cc(C=O)c2ccccc12
InChI
1S/C10H9NO/c1-11-6-8(7-12)9-4-2-3-5-10(9)11/h2-7H,1H3
KXYBYRKRRGSZCX-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
1-Methylindole-3-carboxaldehyde is a heterocyclic indole aldehyde. 1-Methylindole-3-carboxaldehyde on condensation with 2-hydroxybenzohydrazide yields Schiff base.
Applicazioni
1-Methylindole-3-carboxaldehyde may be used in the synthesis of (Z)-3-(1-methyl-1H-indol-3-yl)-2-(thiophen-3-yl)acrylonitrile, via base-catalyzed condensation with thiophene-3-acetonitrile. It was also used in the preparation of monomer, required for the synthesis of poly(3-vinyl-1-methylindole).
- Reactant for preparation of nitroolefins and β-nitroalcohols via microwave- or ultrasound-assisted Henry reactions
- Reactant for synthesis of quinolinones via three-component Ugi reaction
- Reactant for synthesis of α-ketoamides as inhibitors of Dengue virus protease with antiviral activity in cell-culture
- Reactant for preparation of thiazolopyrimidinones as inhibitors of Bcl-2 proteins
- Reactant for preparation of vinylindoles via Peterson olefination or olefination with Nysted reagent
- Reactant for preparation of indolyl alkenes from microwave-enhanced Knoevenagel condensation as antibacterial agents
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Vijayakumar N Sonar et al.
Acta crystallographica. Section C, Crystal structure communications, 60(Pt 3), o217-o218 (2004-03-09)
The title compound, C16H12N2S, has been synthesized by base-catalyzed condensation of 1-methylindole-3-carboxaldehyde with thiophene-3-acetonitrile. The product assumes an approximately planar Z configuration. The molecule has a thienyl-ring flip disorder.
Preliminary analysis of the 1H-and 13C-NMR spectra of poly (3-vinyl-1-methylindole).
Trumbo DL.
Polymer Bull., 37(1), 75-80 (1996)
Wagee A Yehye et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 9), o1824-o1824 (2008-01-01)
In the crystal structure of the title Schiff-base, C(20)H(21)N(3)O(4), the amino group forms an N-H⋯O hydrogen bond to the acetyl group of an adjacent mol-ecule, forming a zigzag chain. The 2-hydr-oxy group is inter-nally hydrogen bonded to the amido group
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.