513830
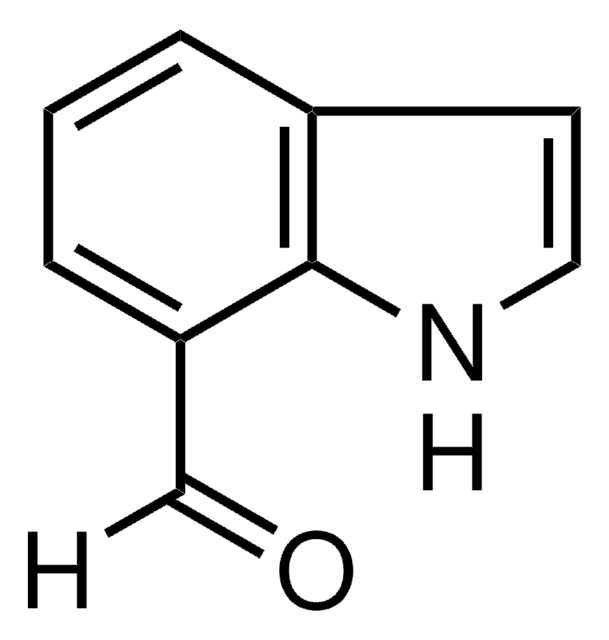
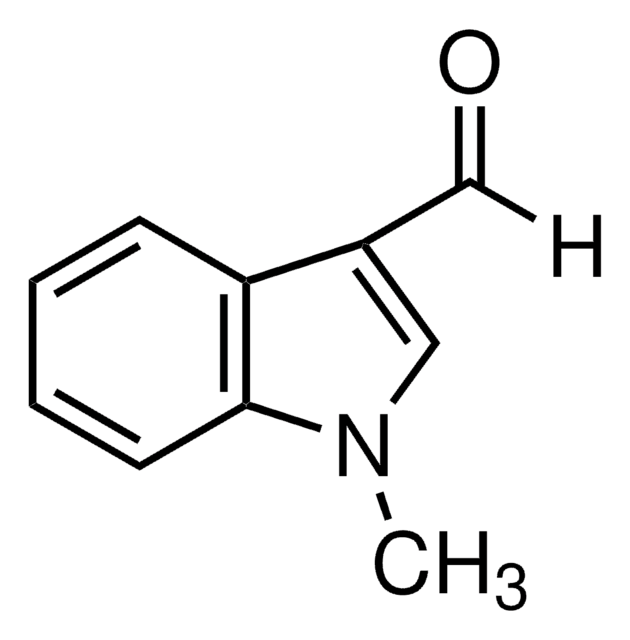
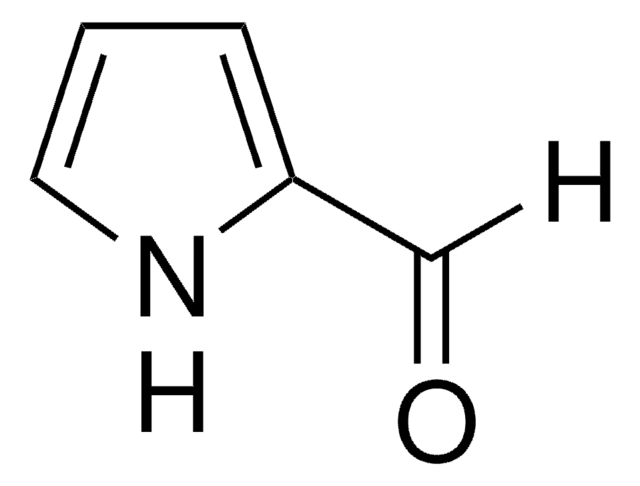
Indole-5-carboxaldehyde
98%
Sinonimo/i:
5-Formylindole
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H7NO
Numero CAS:
Peso molecolare:
145.16
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Punto di fusione
100-103 °C (lit.)
Gruppo funzionale
aldehyde
Stringa SMILE
O=Cc1ccc2[nH]ccc2c1
InChI
1S/C9H7NO/c11-6-7-1-2-9-8(5-7)3-4-10-9/h1-6,10H
ADZUEEUKBYCSEY-UHFFFAOYSA-N
Categorie correlate
Applicazioni
Indole-5-carboxaldehyde can be used as a reactant in the:
- Preparation of curcumin derivatives as anti-proliferative & anti-inflammatory agents
- Preparation of analogs of botulinum neurotoxin serotype A protease inhibitors
- Stereoselective synthesis of dibenzylideneacetone derivatives as β-amyloid imaging probes
- Synthesis of para-para stilbenophanes by McMurry coupling
- Stereoselective synthesis of heteroaromatic (E)-α,β-unsaturated ketones from aldehydes
- Structure-based drug design of aurora kinase A inhibitors
- Preparation of 5-indolyl linked 15- and 18-membered azacrown ethers to study their cation-π interactions.
- Preparation of bibenzimidazole derivatives substituted 5-indolyl moiety in the study of inhibition of topoisomerase I activity.
- Synthesis of (5-(4-(3,4,5-trimethoxybenzoyl)-1H-imidazol-2-yl)-1H-indol-2-yl)(3,4,5-trimethoxyphenyl)methanone and radioiodinated indolochalcone.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Raquel Álvarez et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(12), 3406-3419 (2011-02-24)
The synthesis of a new family of methoxy-substituted [2.7]- and [2.8]paracyclophanes linked by 3-oxapentamethylene-1,5-dioxy and hexamethylene-1,6-dioxy bridges has been carried out by using the McMurry methodology. Related indole compounds were also synthesised. Olefin-to-diol ratios depended on the bridge length, the
Balducci, E.; et al.
European Journal of Organic Chemistry, 311-311 (2011)
Houlihan WJ.
The Chemistry of Heterocyclic Compounds, 367-367 (2009)
Mohane Selvaraj Coumar et al.
Journal of medicinal chemistry, 52(4), 1050-1062 (2009-01-15)
Aurora kinases have emerged as attractive targets for the design of anticancer drugs. Through structure-based virtual screening, novel pyrazole hit 8a was identified as Aurora kinase A inhibitor (IC(50) = 15.1 microM). X-ray cocrystal structure of 8a in complex with
Petr Capek et al.
ACS chemical neuroscience, 2(6), 288-293 (2011-07-12)
Botulinum neurotoxin (BoNT), the etiological agent that causes the neuroparalytic disease botulism, has become a highly studied drug target in light of the potential abuse of this toxin as a weapon of bioterrorism. In particular, small molecule inhibitors of the
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 513830-5G | 4061832492667 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.