511129
1-Methylindole-2-carboxaldehyde
97%
Sinonimo/i:
2-Formyl-1-methylindole, NSC 106285
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H9NO
Numero CAS:
Peso molecolare:
159.18
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Punto di fusione
81-85 °C (lit.)
Gruppo funzionale
aldehyde
Stringa SMILE
Cn1c(C=O)cc2ccccc12
InChI
1S/C10H9NO/c1-11-9(7-12)6-8-4-2-3-5-10(8)11/h2-7H,1H3
IBNGPIOSWCMJGG-UHFFFAOYSA-N
Applicazioni
1-Methylindole-2-carboxaldehyde may be used in the synthesis of indole-based melatonin analog hydrazone derivatives and (E)-5-((benzyloxy)methyl)-5-(((tert-butyldiphenylsilyl)oxy)methyl)-3-((1-methyl-1H-indol-2-yl)methylene)dihydrofuran-2(3H)-one.
Reactant for preparation of:
- Deazapurine isosteres from acylindoles via pyridine ring annulation
- 2,4-dichlorocinnamohydroxamic acid analogs for enhancing pharmacokinetics of botulinum neurotoxin serotype A protease inhibitors
- Tetrahydrocarbazoles via organocatalytic cascade Friedel-Crafts alkylation/Michael addition/aromatization reaction
- Azides, imines and amines via flow processes of amines and trimethylsilyl azide and aza-Wittig reaction
- 3-indolylpyridinedicarbonitriles as anti-inflammatory agents
- Bis(indolyl)methanes as antimicrobial and antioxidant agents
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Sibel Suzen et al.
Journal of enzyme inhibition and medicinal chemistry, 28(6), 1143-1155 (2012-09-22)
Melatonin (MLT) is a strong free-radical scavenger, which protects the body from the effects of oxidants. In recent years, MLT have been described resulting in much attention in the development of synthetic compounds possessing. As a part of our ongoing
Noga Gal et al.
Chembiochem : a European journal of chemical biology, 12(15), 2331-2340 (2012-10-30)
N-methyl-substituted diacylglycerol-indololactones (DAG-indololactones) are newly synthesized effectors of protein kinase C (PKC) isoforms and exhibit substantial selectivity between RasGRP3 and PKCα. We present a comprehensive analysis of membrane interactions and biological activities of several DAG-indololactones. Translocation and binding activity assays
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 511129-5G | 4061832491110 |
| 511129-25G | 4061832841724 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.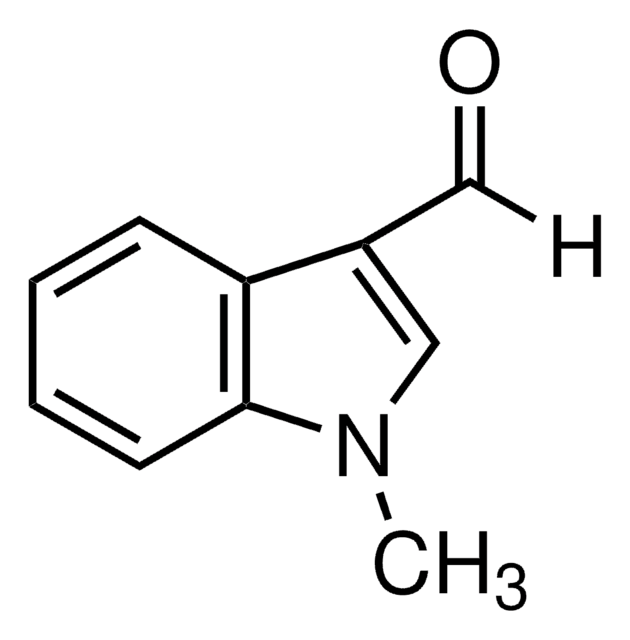

![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)