488712
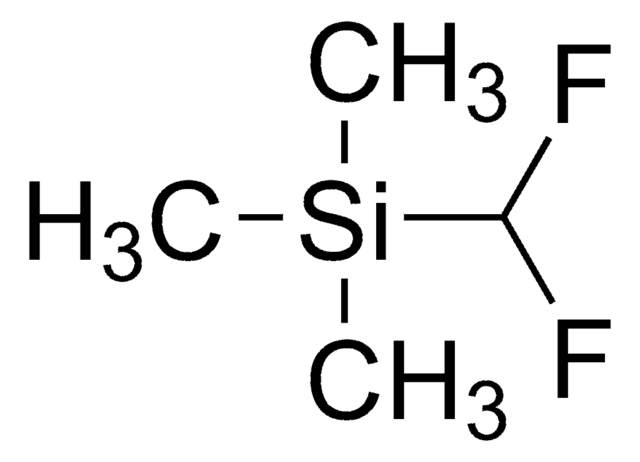
Trimethyl(trifluoromethyl)silane
99%
Sinonimo/i:
(Trifluoromethyl)trimethylsilane, Ruppert’s Reagent, TFMTMS
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
99%
Impiego in reazioni chimiche
reaction type: C-C Bond Formation
P. eboll.
54-55 °C (lit.)
Densità
0.962 g/mL at 20 °C (lit.)
Gruppo funzionale
fluoro
Temperatura di conservazione
2-8°C
Stringa SMILE
C[Si](C)(C)C(F)(F)F
InChI
1S/C4H9F3Si/c1-8(2,3)4(5,6)7/h1-3H3
MWKJTNBSKNUMFN-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Conversion of N-(tert-butylsulfinyl)-imines to trifluoromethylated amines
- Conversion of trans-enones to trans-α-trifluoromethyl silyl ethers
- Trifluoromethylation of azomethine imines
- Conversion of H-phosphonates to CF3-phosphonates
- Nucleophilic addition of the trifluoromethyl group to aldehydes and ketones.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Flam. Liq. 2 - Water-react 2
Codice della classe di stoccaggio
4.3 - Hazardous materials which set free flammable gases upon contact with water
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
1.4 °F - closed cup
Punto d’infiammabilità (°C)
-17 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.