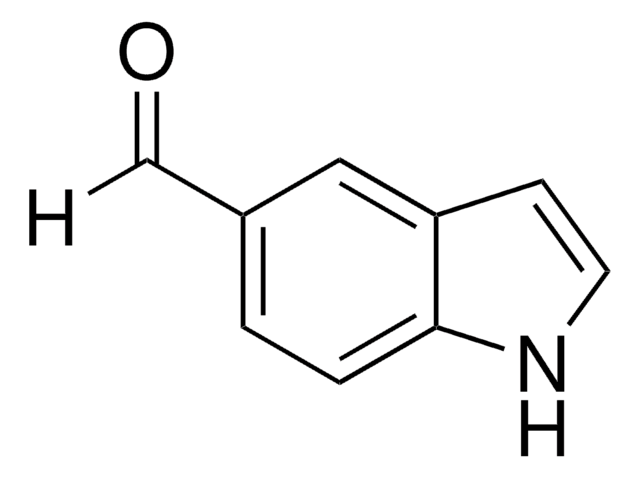
M14943
5-Methoxyindole-3-carboxaldehyde
≥99%
Sinonimo/i:
3-Formyl-5-methoxyindole, NSC 521754
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H9NO2
Numero CAS:
Peso molecolare:
175.18
Beilstein:
132769
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
≥99%
Punto di fusione
179-183 °C (lit.)
Stringa SMILE
COc1ccc2[nH]cc(C=O)c2c1
InChI
1S/C10H9NO2/c1-13-8-2-3-10-9(4-8)7(6-12)5-11-10/h2-6,11H,1H3
TUWARWGEOHQXCO-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- reactant in synthesis of tryptophan dioxygenase inhibitors as potential anticancer immunomodulators
- reactant in preparation of inhibitor of the C-terminal domain of RNA polymerase II
- reactant in preparation of imidazopyridines and imidazobenzothiazoles
- reactant in preparation of fluorescent neuroactive probes for brain imaging
- reactant in preparation of antibacterial agents
- reactant in synthesis of antiandrogens
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Jinming Zhou et al.
Investigational new drugs, 28(3), 291-298 (2009-04-25)
A crucial event in prostate cancer progression is the transition from a hormone-sensitive to a lethal castration-refractory disease state. The antagonist-to-agonist conversion due to mutation in AR is a critical problem with the current clinically used antiandrogens. We aim to
Adrienne S Brown et al.
Organic & biomolecular chemistry, 9(7), 2142-2148 (2011-02-05)
A set of spectrally diverse stilbazolium dyes was identified in an uptake assay using cultured brainstem and cerebellum cells isolated from e19 chicks. Pretreatment of cells with indatraline, a monoamine reuptake inhibitor, allowed identification of dyes that may interact with
Shuhong Wu et al.
Journal of medicinal chemistry, 54(8), 2668-2679 (2011-03-30)
To optimize the antitumor activity of oncrasin-1, a small molecule RNA polymerase II inhibitor, we evaluated 69 oncrasin-1 analogues for their cytotoxic activity against normal human epithelial cells and K-Ras mutant tumor cells. About 40 of those compounds were as
Taleb H Al-Tel et al.
European journal of medicinal chemistry, 46(5), 1874-1881 (2011-03-19)
New antimicrobial agents, imidazo[1,2-a]pyridine and imidazo[2,1-b][1,3]benzothiazole, have been synthesized. Their antimicrobial activities were conducted against various Gram-positive, Gram-negative bacteria and fungi. Compounds 6c, 7a, 10b, 11a, 12b, 14a, 14b, 15a and 15b, exerted strong inhibition of the investigated bacterial and
Anas J M Rasras et al.
European journal of medicinal chemistry, 45(6), 2307-2313 (2010-02-26)
Synthesis and antimicrobial activity of cholic acid analogues 4a-t are reported. The synthesis of 4a-t was accomplished from ethylcholate 2. The hydrazone moiety was introduced via coupling of the cholic acid hydrazide (3) with appropriately functionalized aldehyde utilizing acetic acid
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.


![1H-Benzo[g]indole 97%](/deepweb/assets/sigmaaldrich/product/structures/568/798/abc69b41-4c75-4dce-8e3a-b6ff7851c6fd/640/abc69b41-4c75-4dce-8e3a-b6ff7851c6fd.png)