518743
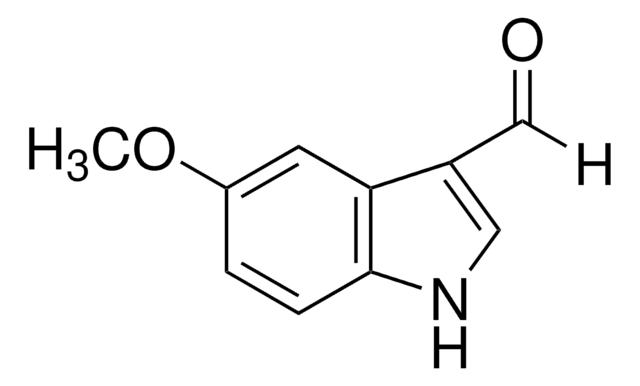
5-Bromoindole-3-carboxaldehyde
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H6BrNO
Numero CAS:
Peso molecolare:
224.05
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Punto di fusione
204-207 °C (lit.)
Gruppo funzionale
aldehyde
bromo
Stringa SMILE
[H]C(=O)c1c[nH]c2ccc(Br)cc12
InChI
1S/C9H6BrNO/c10-7-1-2-9-8(3-7)6(5-12)4-11-9/h1-5,11H
PEENKJZANBYXNB-UHFFFAOYSA-N
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Sung Dae Cho et al.
Molecular cancer therapeutics, 7(7), 2109-2120 (2008-07-23)
Bis(3'-indolyl)methane (DIM) is a metabolite of the phytochemical indole-3-carbinol, and both compounds exhibit a broad spectrum of anticancer activities. We have developed a series of synthetic symmetrical ring-substituted DIM analogues, including 5,5'-dibromoDIM, which are more potent than DIM as inhibitors
S J Wratten et al.
Antimicrobial agents and chemotherapy, 11(3), 411-414 (1977-03-01)
An antibiotic-producing pseudomonad was isolated from a seawater sample from a La Jolla, Calif., tidepool. The pseudomonad produces two novel antibacterial compounds, 2-n-pentyl-4-quinolinol and 2-n-heptyl-4-quinolinol. It also synthesizes indole-3-carboxaldehyde, 6-bromoindole-3-carboxaldehyde, and the known antibiotic p-hydroxybenzaldehyde. Each of these compounds was
Elizabeth Almeida Lafayette et al.
European journal of medicinal chemistry, 136, 511-522 (2017-05-23)
Molecules bearing indole nucleus present diverse biological properties such as antitumor and anti-inflammatory activities that can be associated both to DNA and protein interactions. This study focused on the synthesis of new indole derivatives with thiazolidines and imidazolidine rings condensed
Ming-Zhi Zhang et al.
European journal of medicinal chemistry, 92, 776-783 (2015-01-31)
Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.