275034
9,10-Phenanthrenequinone
95%
Sinonimo/i:
9,10-Phenanthrenedione
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C14H8O2
Numero CAS:
Peso molecolare:
208.21
Beilstein:
608838
Numero CE:
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.23
Prodotti consigliati
Saggio
95%
Stato
powder
Punto di fusione
209-212 °C (lit.)
Stringa SMILE
O=C1C(=O)c2ccccc2-c3ccccc13
InChI
1S/C14H8O2/c15-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14(13)16/h1-8H
YYVYAPXYZVYDHN-UHFFFAOYSA-N
Informazioni sul gene
human ... PTPN1(5770) , PTPRC(5788)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
The quinones of polycyclic aromatic hydrocarbons are present in abundance in all burnt organic material. On being used to passivate silicon surfaces, it reacts with the dangling bonds on the surface via a heteroatomic Diels-Alder reaction. On account of the Π-electron conjugation, the semi conducting nature of the silicon is unaffected.
Applicazioni
9,10-Phenanthrenequinon may be used for high quality passivation on silicon (100) surfaces. Quinones may serve as substrates for a variety of flavoenzymes.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Acute 1 - Eye Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
473.0 °F
Punto d’infiammabilità (°C)
245 °C
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
P L Chesis et al.
Proceedings of the National Academy of Sciences of the United States of America, 81(6), 1696-1700 (1984-03-01)
The mutagenicity of various quinones, a class of compounds widely distributed in nature, is demonstrated in the Salmonella TA104 tester strain. The metabolic pathways by which four quinones, menadione, benzo[a]pyrene 3,6-quinone, 9,10-phenanthrenequinone, and danthron, caused mutagenicity in this test system
Electronic structure and band alignment of 9, 10-phenanthrenequinone passivated silicon surfaces
Avasthi, Sushobhan, et al.
Surface Science, 605(13), 1308-1312 (2011)
Petr Milko et al.
Inorganic chemistry, 48(24), 11734-11742 (2009-11-26)
With the use of the model complexes [(PQ)FeCl(CH(3)O)](+), [(phen)FeCl(CH(3)O)](+), and [(PQ)(phen)FeCl(CH(3)O)](+), where PQ is 9,10-phenanthraquinone and phen is 1,10-phenanthroline, the reactivity of phenanthraquinone in complexes with iron(III) is investigated. It is shown that 9,10-phenanthraquinone takes part in redox processes occurring
Naoya Kishikawa et al.
Talanta, 85(1), 809-812 (2011-06-08)
9,10-Phenanthrenequinone (PQ) is harmful environmental pollutant that is detected in airborne particulates. The measurement of PQ in the air should be necessary to evaluate the potential adverse effects of PQ on human health. We have recently developed a determination method
Michael C Byrns et al.
Biochemical pharmacology, 75(2), 484-493 (2007-10-24)
Aldo-keto reductase (AKR) 1C3 (type 2 3alpha-HSD, type 5 17beta-HSD, and prostaglandin F synthase) regulates ligand access to steroid hormone and prostaglandin receptors and may stimulate proliferation of prostate and breast cancer cells. NSAIDs are known inhibitors of AKR1C enzymes.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.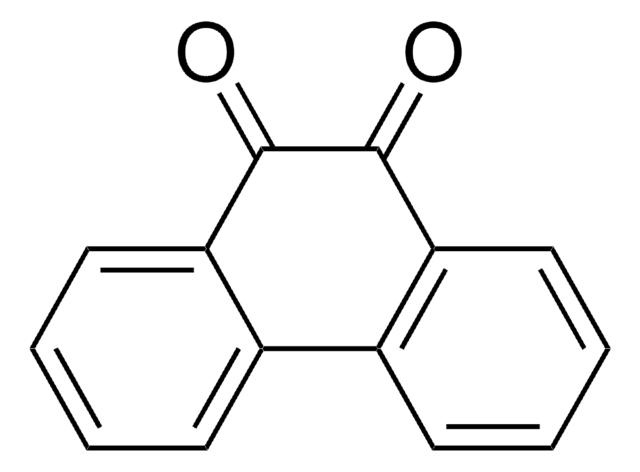





![6H-Benzo[cd]pyren-6-one BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/121/467/11adf097-4f11-4b40-a73f-910f36624e9c/640/11adf097-4f11-4b40-a73f-910f36624e9c.png)