249505
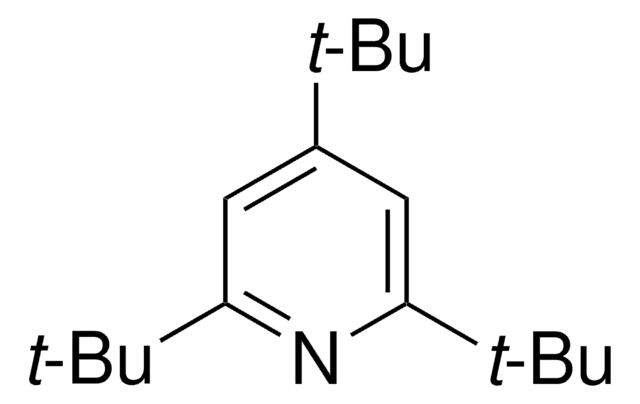
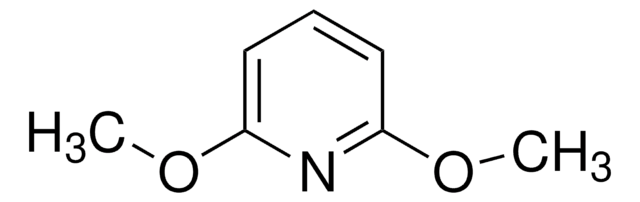
2,6-Di-tert-butyl-4-methylpyridine
98%
Sinonimo/i:
2,6-Bis(1,1-dimethylethyl)-4-methylpyridine, 2,6-Bis(tert-butyl)-4-methylpyridine, 2,6-Ditert-butyl-4-methylpyridine, 4-Methyl-2,6-di-tert-butylpyridine
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
solid
Indice di rifrazione
n20/D 1.4763 (lit.)
P. ebollizione
233 °C (lit.)
Punto di fusione
33-36 °C (lit.)
Solubilità
ethanol: soluble 5%, clear to slightly hazy, colorless to dark yellow
Temperatura di conservazione
2-8°C
Stringa SMILE
Cc1cc(nc(c1)C(C)(C)C)C(C)(C)C
InChI
1S/C14H23N/c1-10-8-11(13(2,3)4)15-12(9-10)14(5,6)7/h8-9H,1-7H3
HVHZEKKZMFRULH-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
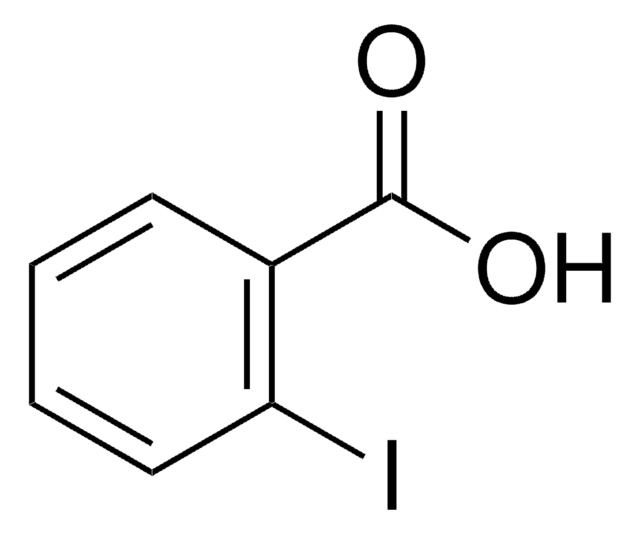
- in the synthesis of 1,2-dihydro-2-silanaphthalene derivatives
- as base in PtCl4-catalyzed cyclization reactions of homopropargyl azide derivatives
- diastereoselective synthesis of β-thiomannopyranosides
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
183.2 °F - closed cup
Punto d’infiammabilità (°C)
84 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 249505-25G | 4061833070819 |
| 249505-1G | 4061825928623 |
| 249505-1KG | |
| 249505-5G | 4061825928630 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.