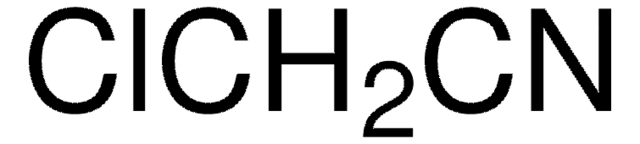157759
Sulfur monochloride
98%
Sinonimo/i:
Sulfur chloride
About This Item
Prodotti consigliati
Grado
for analytical purposes
Livello qualitativo
Densità del vapore
4.7 (vs air)
Tensione di vapore
6.8 mmHg ( 20 °C)
Saggio
98%
Stato
liquid
Temp. autoaccensione
451 °F
Indice di rifrazione
n20/D 1.658 (lit.)
P. ebollizione
138 °C (lit.)
Punto di fusione
−80 °C (lit.)
Densità
1.688 g/mL at 25 °C (lit.)
Stringa SMILE
ClSSCl
InChI
1S/Cl2S2/c1-3-4-2
PXJJSXABGXMUSU-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- To synthesize functionalized aryl and heteroaryl disulfides from functionalized zinc organometallics.
- As a reagent to prepare heterocycles with various numbers of sulfur atoms.
- As a cross-linking agent to prepare rubber aerogels with enhanced thermal stability.
- Toprepare macroporous cryogels of polyisobutylene and silica nanoparticles.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Skin Corr. 1A
Rischi supp
Codice della classe di stoccaggio
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Colloidal quantum dots (CQDs) are semiconducting crystals of only a few nanometers (ca. 2–12 nm) coated with ligand/surfactant molecules to help prevent agglomeration.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.