推荐产品
等級
pharmaceutical primary standard
API 家族
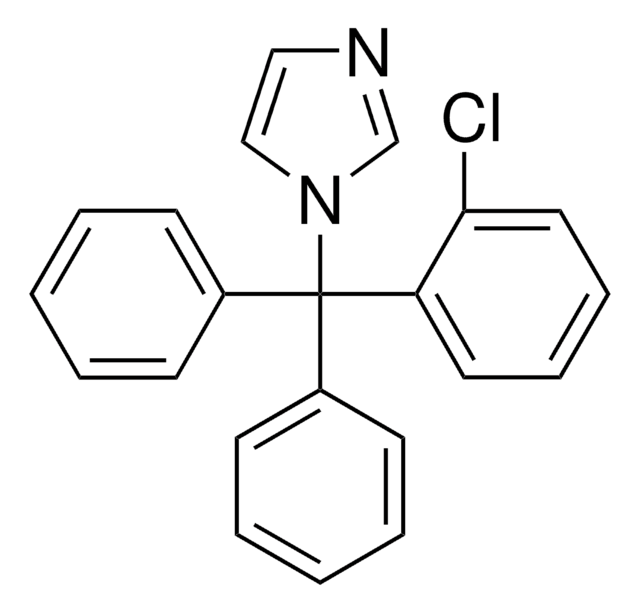
triamcinolone
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
[H][C@@]12CCC3=CC(=O)C=C[C@]3(C)[C@@]1(F)[C@@H](O)C[C@@]4(C)[C@@]2([H])C[C@H]5OC(C)(C)O[C@@]45C(=O)CO
InChI
1S/C24H31FO6/c1-20(2)30-19-10-16-15-6-5-13-9-14(27)7-8-21(13,3)23(15,25)17(28)11-22(16,4)24(19,31-20)18(29)12-26/h7-9,15-17,19,26,28H,5-6,10-12H2,1-4H3/t15-,16-,17-,19+,21-,22-,23-,24+/m0/s1
InChI 密鑰
YNDXUCZADRHECN-JNQJZLCISA-N
基因資訊
human ... NR3C1(2908)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Triamcinolone acetonide USP reference standard intended for use in specified quality tests and assays.
Also used to prepare system suitability, standard, and standard stock solution for assay, impurity analysis, and performance tests according to the given below monographs of United States Pharmacopeia (USP):
Also used to prepare system suitability, standard, and standard stock solution for assay, impurity analysis, and performance tests according to the given below monographs of United States Pharmacopeia (USP):
- Triamcinolone Hexacetonide
- Triamcinolone Acetonide Injectable Suspension
- Triamcinolone Acetonide Nasal Spray
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Taygan Yilmaz et al.
Ophthalmology, 116(5), 902-911 (2009-05-05)
To compare intravitreal triamcinolone acetonide (IVTA) injection versus no treatment or sub-Tenon triamcinolone acetonide (STTA) injection in improving visual acuity (VA) of patients with refractory diabetic macular edema (DME; unresponsive to focal laser therapy). Diabetic macular edema is the leading
Daniel I Rhon et al.
Annals of internal medicine, 161(3), 161-169 (2014-08-05)
Corticosteroid injections (CSIs) and physical therapy are used to treat patients with the shoulder impingement syndrome (SIS) but have never been directly compared. To compare the effectiveness of 2 common nonsurgical treatments for SIS. Randomized, single-blind, comparative-effectiveness, parallel-group trial. (ClinicalTrials.gov:
Kent W Small et al.
Ophthalmology, 121(4), 952-958 (2014-02-11)
To report a series of cases with fungal endophthalmitis occurring after intravitreal injection of triamcinolone derived from a single lot prepared by a compounding pharmacy. Retrospective, observational case series. Seventeen eyes treated with triamcinolone obtained from a single lot subsequently
Luis A Solchaga et al.
Journal of orthopaedic research : official publication of the Orthopaedic Research Society, 32(1), 145-150 (2013-09-11)
This study compared the effect of intra-tendon (IT) delivery of recombinant human platelet-derived growth factor-BB (rhPDGF-BB), platelet-rich plasma (PRP) and corticosteroids in a rat tendinopathy model. Seven days after collagenase induction of tendinopathy, a 30-µl IT injection was administered. Treatments
C M Jermak et al.
Survey of ophthalmology, 52(5), 503-522 (2007-08-28)
Triamcinolone acetonide has been effectively used in ocular therapeutics for over 50 years. Its use has increased dramatically in recent years for periocular and intraocular treatment of retinal vasculature disease and uveitis. This comprehensive review discusses the pharmacokinetics of triamcinolone
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门