推荐产品
等級
pharmaceutical primary standard
API 家族
lactose
製造商/商標名
USP
應用
cleaning products
cosmetics
food and beverages
personal care
pharmaceutical (small molecule)
形式
neat
SMILES 字串
O.OC[C@@H](O)[C@@H](O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O)[C@H](O)[C@@H](O)C=O
InChI
1S/C12H22O11.H2O/c13-1-4(16)7(18)11(5(17)2-14)23-12-10(21)9(20)8(19)6(3-15)22-12;/h1,4-12,14-21H,2-3H2;1H2/t4-,5+,6+,7+,8-,9-,10+,11+,12-;/m0./s1
InChI 密鑰
HBDJFVFTHLOSDW-XBLONOLSSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
本品按现行药典规定交付。所有为支持本产品而提供的信息,包括SDS和任何产品信息单均由药典颁发机构制定并发布。如需进一步信息和支持,请访问现行药典网站。→
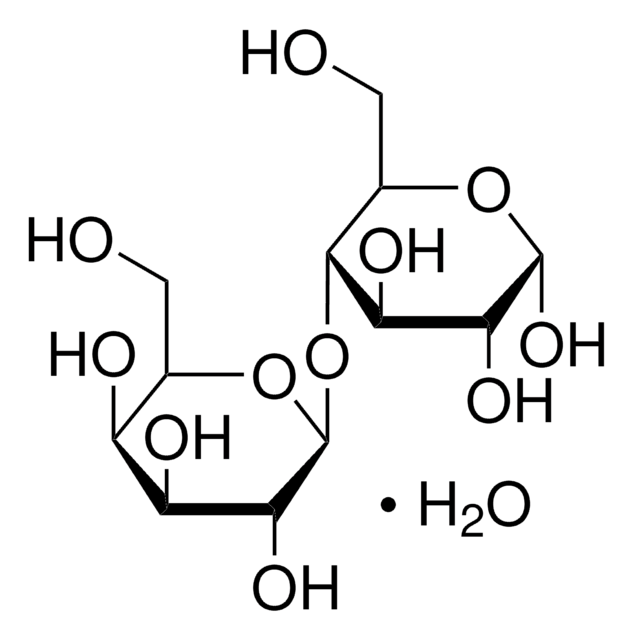
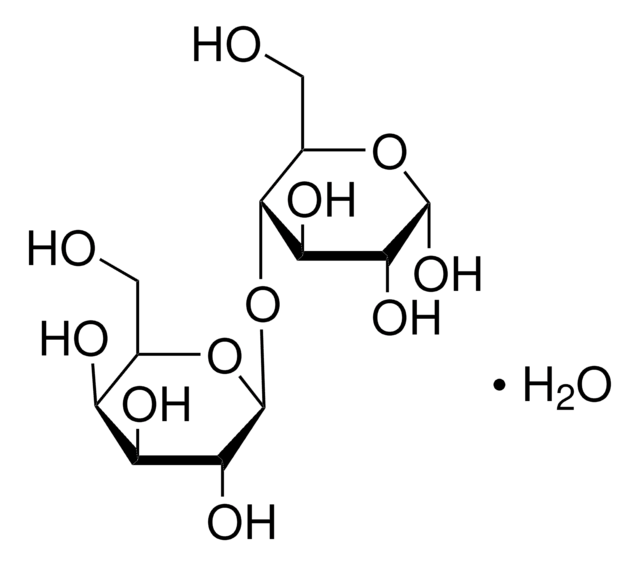
乳糖一水合物是O-β-d-吡喃半乳糖基(1→4)-α-D-吡喃型葡萄糖的一水合物。
乳糖一水合物是O-β-d-吡喃半乳糖基(1→4)-α-D-吡喃型葡萄糖的一水合物。
應用
乳糖一水合物USP参考标准品用于指定质量测试和分析。
还可用于制备标准溶液,用于根据以下美国药典(USP)各论通过薄层色谱(TLC)进行鉴定:
还可用于制备标准溶液,用于根据以下美国药典(USP)各论通过薄层色谱(TLC)进行鉴定:
- 乳糖一水合物
- 半乳糖
分析報告
这些产品仅供测试和分析使用。它们不适用于人类或动物的给药,不可用于诊断、治疗或治愈任何疾病。
其他說明
可能适用相应的销售限制。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Galactose
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 34(3), 2953-2953 (2018)
Tamás Firkala et al.
Journal of pharmaceutical and biomedical analysis, 107, 318-324 (2015-02-01)
This paper reports the application of surface enhanced Raman chemical imaging (SER-CI) as a potentially non-destructive quantitative analytical method for the investigation of model pharmaceutical formulations containing the active pharmaceutical ingredient (API) in low concentrations (0.5-2%). The application of chemometric
Akhtar Siddiqui et al.
Journal of pharmaceutical sciences, 103(9), 2819-2828 (2014-03-04)
The objective of this study was to develop powder X-ray diffraction (XRPD) chemometric model for quantifying crystalline tacrolimus from solid dispersion (SD). Three SDs (amorphous tacrolimus component) with varying drug to excipient ratios (24.4%, 6.7%, and 4.3% drug) were prepared.
J Nijdam et al.
Colloids and surfaces. B, Biointerfaces, 123, 53-60 (2014-09-30)
Segregation of the protein bovine serum albumin (BSA) and lactose in thin aqueous films during drying was investigated by examining the composition of the dried films using inverse micro Raman spectroscopy (IMRS) and X-ray photoelectron spectroscopy (XPS) sputter-depth profiling. The
Naser Tavakoli et al.
Journal of microencapsulation, 31(6), 529-534 (2014-04-05)
Repaglinide, an oral antidiabetic agent, has a rapid onset of action and short half-life of approximately 1 h. Designing a controlled release dosage form of the drug is required to maintain its therapeutic blood level and to eliminate its adverse effects
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门