推荐产品
生物源
synthetic (organic)
品質等級
化驗
≥98% (TLC)
形狀
powder
溶解度
hot water: 19.60-20.40 mg/mL, clear, colorless
儲存溫度
2-8°C
SMILES 字串
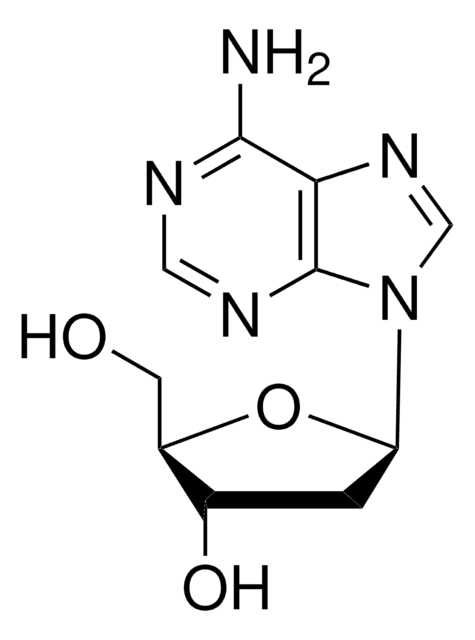
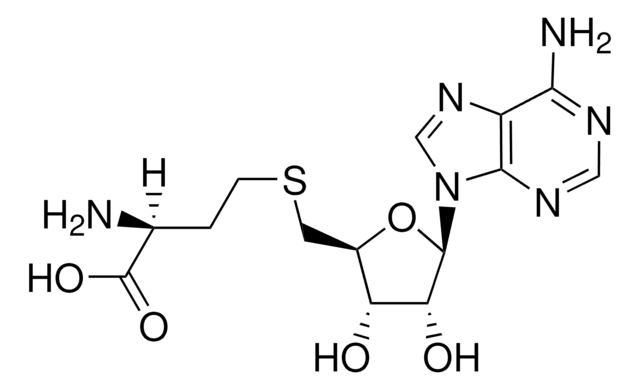
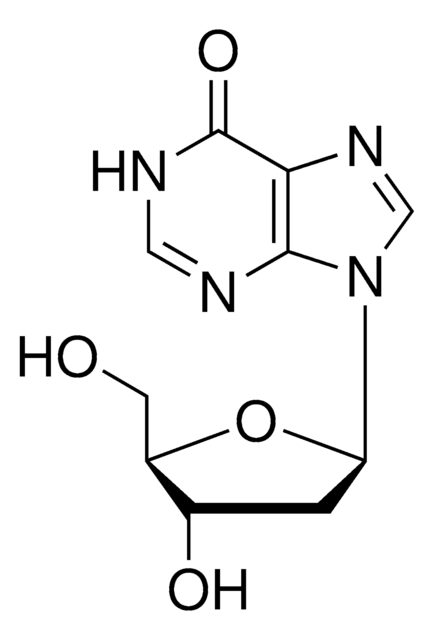
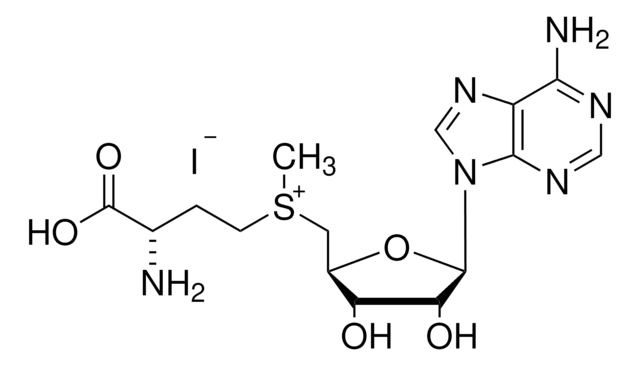
C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C10H13N5O3/c1-4-6(16)7(17)10(18-4)15-3-14-5-8(11)12-2-13-9(5)15/h2-4,6-7,10,16-17H,1H3,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1
InChI 密鑰
XGYIMTFOTBMPFP-KQYNXXCUSA-N
正在寻找类似产品? 访问 产品对比指南
應用
5′-脱氧腺苷已用于:
- 作为质谱中的标准品
- 、作为筛选胸苷磷酸化酶活性的抑制剂
- 、作为 5′-脱氧腺苷脱氨酶(DadD)试验的底物
生化/生理作用
5′-脱氧腺苷是微生物中甲基硫代腺苷/ S -腺苷同型半胱氨酸 (MTA/SAH)核苷酶的底物。 5′-脱氧腺苷是 S -腺苷甲硫氨酸(SAM)裂解的副产物。5′ 高水平脱氧腺苷抑制SAM 依赖性酶。它还抑制生物素合成酶(BioB)和硫辛酸合成酶(LipA)。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
The nucleoside derivative 5?-O-trityl-inosine (KIN59) suppresses thymidine phosphorylase-triggered angiogenesis via a noncompetitive mechanism of action
Liekens S, et al.
Test, 279(28), 29598-29605 (2004)
Kenichi Yokoyama et al.
Biochemistry, 47(34), 8950-8960 (2008-08-05)
BtrN is a radical SAM ( S-adenosyl- l-methionine) enzyme that catalyzes the oxidation of 2-deoxy- scyllo-inosamine (DOIA) into 3-amino-2,3-dideoxy- scyllo-inosose (amino-DOI) during the biosynthesis of 2-deoxystreptamine (DOS) in the butirosin producer Bacillus circulans. Recently, we have shown that BtrN catalyzes
Charles J Walsby et al.
Inorganic chemistry, 44(4), 727-741 (2005-04-30)
Electron paramagnetic resonance (EPR), electron-nuclear double resonance (ENDOR), and Mössbauer spectroscopies and other physical methods have provided important new insights into the radical-SAM superfamily of proteins, which use iron-sulfur clusters and S-adenosylmethionine to initiate H atom abstraction reactions. This remarkable
Joseph T Jarrett
Current opinion in chemical biology, 7(2), 174-182 (2003-04-26)
Adenosylmethionine-dependent radical enzymes provide a novel mechanism for generating the highly oxidizing 5'-deoxyadenosyl radical in an anaerobic reducing environment. Recent studies suggest a unique covalent interaction between adenosylmethionine and a catalytic iron-sulfur cluster that may promote inner-sphere electron transfer to
Gunhild Layer et al.
Current opinion in chemical biology, 8(5), 468-476 (2004-09-29)
'Radical SAM' enzymes juxtapose a [4Fe-4S] cluster and S-adenosyl-l-methionine (SAM) to generate catalytic 5'-deoxyadenosyl radicals. The crystal structures of oxygen-independent coproporphyrinogen III oxidase HemN and biotin synthase reveal the positioning of both cofactors with respect to each other and relative
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门