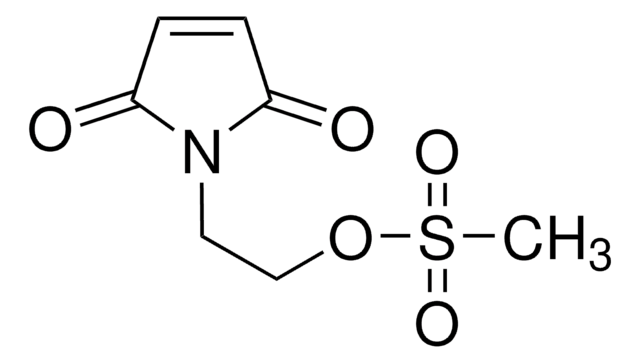
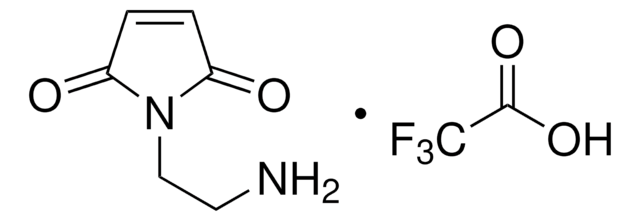
推荐产品
應用
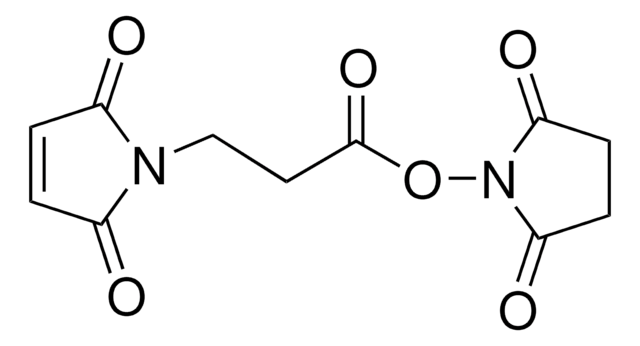
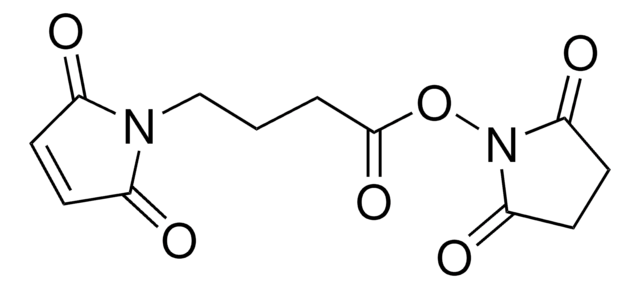
Gamma-maleimidobutyric acid may be used as a spacer in the construction of drug and other types of bioconjugates. 4-Maleimidobutyric acid is used with N-hydroxysuccinimide ester as a bifunctional cross-linking agent.
Modification reagent for thiol groups in proteins
其他說明
SH-label for the modification of peptides and proteins; Used for the preparation of a new bleomycin analog for enzyme immunoassay (EIA); probe for membrane SH-groups
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
K Fujiwara et al.
Cancer research, 41(10), 4121-4126 (1981-10-01)
An antibody directed toward pepleomycin, a new antitumor antibiotic related structurally to bleomycin, has been produced in rabbits by immunization with a pepleomycin-protein conjugate which was prepared by a novel procedure of coupling pepleomycin to mercaptosuccinylated bovine serum albumin using
Biochemical Society Transactions, 11, 753-753 (1983)
N-polymethylenecarboxymaleimides -- a new class of probes for membrane sulphydryl groups.
D G Griffiths et al.
FEBS letters, 134(2), 261-263 (1981-11-16)
Marie Pribylova et al.
International journal of pharmaceutics, 415(1-2), 175-180 (2011-06-15)
A new targeted conjugates in which paclitaxel was used as a cytostatic compound and an analog of the gonadotropin-releasing hormone (GnRH) as a targeting moiety were synthesized. The molecule of the peptide hormone GnRH was modified to allow its connection
Weibo Cai et al.
Nature protocols, 3(1), 89-96 (2008-01-15)
To take full advantage of the unique optical properties of quantum dots (QDs) and expedite future near-infrared fluorescence (NIRF) imaging applications, QDs need to be effectively, specifically and reliably directed to a specific organ or disease site after systemic administration.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门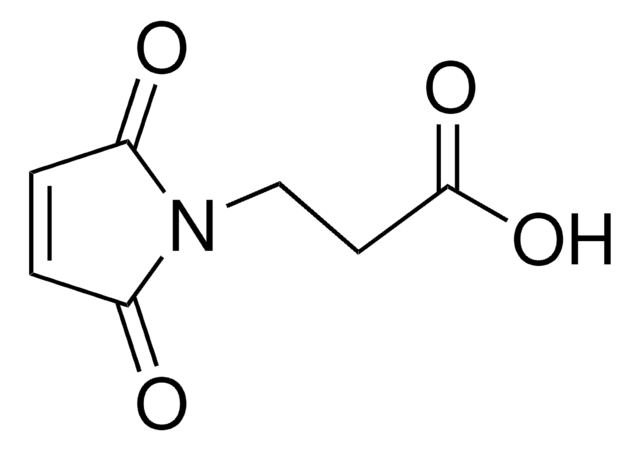

![O-[2-(Boc-amino)ethyl]-O′-(2-maleimidoethyl)ethylene glycol ≥96.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/270/188/cd3145b1-0ad3-409b-85c2-73937e007f04/640/cd3145b1-0ad3-409b-85c2-73937e007f04.png)