推荐产品
品質等級
化驗
≥98.5% (HPLC)
形狀
solid
反應適用性
reagent type: linker
mp
167-171 °C
官能基
NHS ester
儲存溫度
2-8°C
SMILES 字串
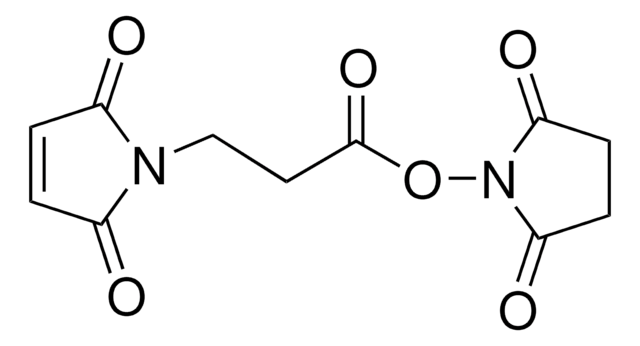
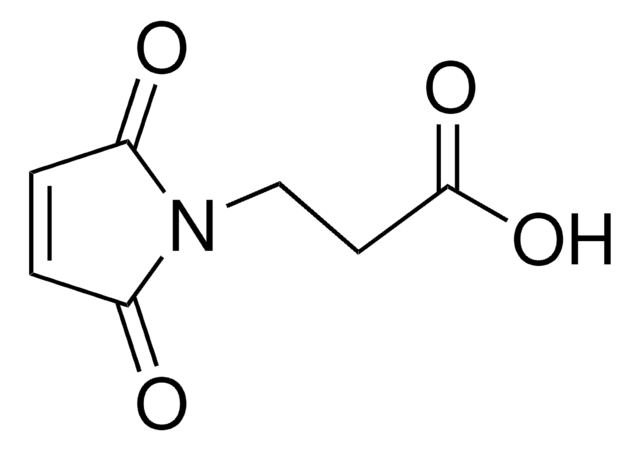
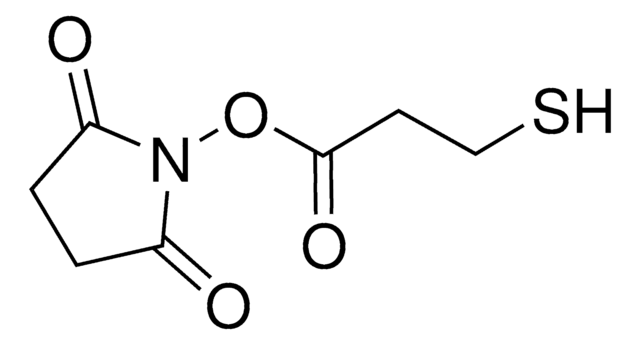
O=C1CCC(N1OC(CCN2C(C=CC2=O)=O)=O)=O
InChI
1S/C11H10N2O6/c14-7-1-2-8(15)12(7)6-5-11(18)19-13-9(16)3-4-10(13)17/h1-2H,3-6H2
InChI 密鑰
JKHVDAUOODACDU-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
琥珀酰亚胺基 3-马来酰亚胺丙酸酯 (SMP,BMPS) 用作脂肪族异双功能交联剂试剂。用于诸如酶免疫测定前半抗原与酶交联或荧光底物与肽抗原交联等技术,用于荧光免疫测定。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
S J Xiao et al.
Journal of materials science. Materials in medicine, 8(12), 867-872 (2004-09-07)
Surface modification of acid-pretreated titanium with 3-aminopropyltriethoxylsilane (APTES) in dry toluene resulted in covalently bonded siloxane films with surface coverage that was relatively controllable by regulating the reaction conditions. A hetero-bifunctional cross-linker, N-succinimidyl-3-maleimidopropionate (SMP), reacted with the terminal amino groups
T. Kitagawa
Enzyme Immunoassay, 81-81 (1981)
S Heyse et al.
Protein science : a publication of the Protein Society, 4(12), 2532-2544 (1995-12-01)
A new method is presented for measuring sensitively the interactions between ligands and their membrane-bound receptors in situ using integrated optics, thus avoiding the need for additional labels. Phospholipid bilayers were attached covalently to waveguides by a novel protocol, which
M C Durrieu et al.
Journal of materials science. Materials in medicine, 15(7), 779-786 (2004-09-28)
Ceramics possess osteoconductive properties but exhibit no intrinsic osteoinductive capacity. Consequently, they are unable to induce new bone formation in extra osseous sites. In order to develop bone substitutes with osteogenic properties, one promising approach consists of creating hybrid materials
Mengjie Rui et al.
International journal of nanomedicine, 12, 217-237 (2017-01-25)
The development of drug resistance in cancer cells is one of the major obstacles to achieving effective chemotherapy. We hypothesized that the combination of a doxorubicin (Dox) prodrug and microRNA (miR)21 inhibitor might show synergistic antitumor effects on drug-resistant breast
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

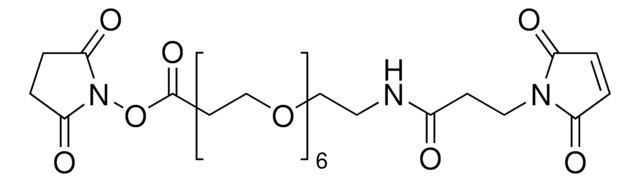
![O-[N-(3-马来酰亚胺丙酰)氨基乙基]-O′-[3-(N-琥珀酰亚氨氧基)-3-氧代丙基]三甘醇 ≥90% (NMR)](/deepweb/assets/sigmaaldrich/product/structures/328/004/054496e8-534d-44d8-a8d7-b85d6377947e/640/054496e8-534d-44d8-a8d7-b85d6377947e.png)