推荐产品
品質等級
化驗
≥98.0% (HPLC)
形狀
solid
反應適用性
reagent type: linker
mp
67-73 °C
70-73 °C (lit.)
官能基
NHS ester
儲存溫度
−20°C
SMILES 字串
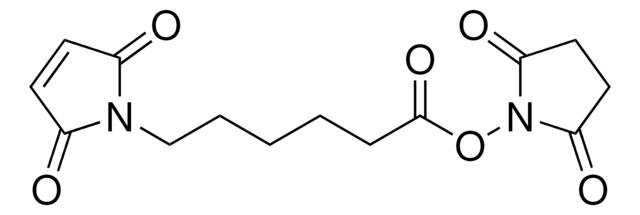
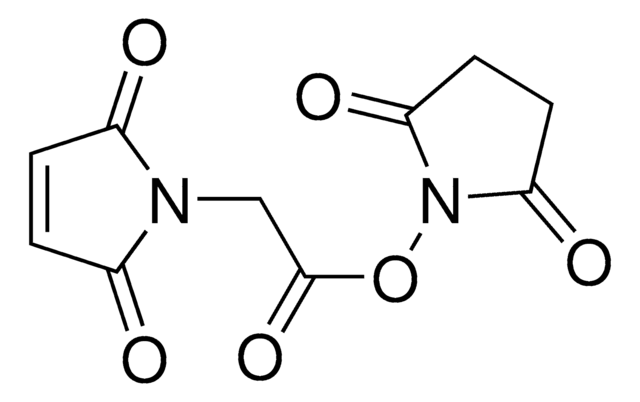
O=C(ON(C(CC1)=O)C1=O)CCCCCN2C(C=CC2=O)=O
InChI
1S/C14H16N2O6/c17-10-5-6-11(18)15(10)9-3-1-2-4-14(21)22-16-12(19)7-8-13(16)20/h5-6H,1-4,7-9H2
InChI 密鑰
VLARLSIGSPVYHX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
6-Maleimidohexanoic acid N-hydroxysuccinimide ester is a heterobifunctional cross-linking reagent with amine and sulfhydryl reactivity. Typically, coupled initially to molecules containing primary amines by amide bonds buffered at pH 7.5 (6.5-8.5). Second coupling specific for molecules containing free sulfhydryl by thioether linkage buffered at pH 6.8 (6.5-7.0). Useful for preparation of enzyme immunoconjugates and hapten carrier molecule conjugates.
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
其他說明
Reagent for cross-linking and immobilisation of proteins
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
T. Kitagawa
Enzyme Immunoassay, 81-81 (1981)
Preparation and characterization of hetero-bifunctional cross-linking reagents for protein modifications.
Kitagawa, T., et al.
Chemical & Pharmaceutical Bulletin, 29, 1130-1130 (1981)
Mitsuko Maeda et al.
Bioorganic & medicinal chemistry letters, 15(3), 621-624 (2005-01-25)
The adenovirus vector is a promising carrier for the efficient transfer of genes into cells via the coxackie-adenovirus receptor (CAR) and integrins (alphavbeta3 and alphavbeta5). The clinical use of the adenovirus vector remains problematic however. Successful administration of this vector
Shinya Kida et al.
Chemical & pharmaceutical bulletin, 55(4), 685-687 (2007-04-06)
6-maleimidohexanoic acid N-hydroxysuccinimide ester has been used widely for preparation of enzyme immunoconjugates as a unique heterobifunctional cross-linking reagent. Its heterobifunctional reactivity is good, but its ester portion hydrolyzes easily in the presence of water. Several 6-maleimidohexanoic acid active esters
Y Nakano et al.
International archives of allergy and immunology, 120(3), 199-208 (1999-12-11)
We have previously reported that ovalbumin (OVA) coupled with liposome via glutaraldehyde (GA) induced OVA-specific- and IgE-selective unresponsiveness in mice. In this study, OVA-liposome conjugates were made using four different coupling protocols: via GA, N-(6-maleimidocaproyloxy) succinimide (EMCS), disuccinimidyl suberate (DSS)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门