P6534
Phospholipase A2 from porcine pancreas
ammonium sulfate suspension, ≥600 units/mg protein
Sinonimo/i:
Lecithinase A, PLA2, Phosphatidylcholine 2-acylhydrolase
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Prodotti consigliati
Stato
ammonium sulfate suspension
Livello qualitativo
Attività specifica
≥600 units/mg protein
N° accesso UniProt
Temperatura di conservazione
2-8°C
Informazioni sul gene
pig ... PLA2G1B(445525)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
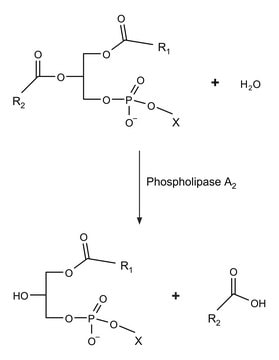
Phospholipase A2 is a small disulfide-rich protein having 124 residues. It is a calcium-dependent enzyme.
Applicazioni
Phospholipase A2 has been used in phospholipase assay and to determine rat renal proximal tubular segments (PTS) viability during oxygenation and hypoxia-reoxygenation.
Azioni biochim/fisiol
Hydrolyzes the β-ester bond of zwitterionic glycerophospholipids. Preferred substrates are phosphatidylcholine, phosphatidylethanolamine, and their plasmalogen analogues. Phosphatidylinositol and phosphatidylserine are also hydrolyzed. Aggressively attacks phospholipids in membranes of intact cells.
It has a high catalytic activity on aggregated substrates compared to monomeric substrates.
Definizione di unità
One unit will hydrolyze 1.0 μmole of soybean L-α-phosphatidylcholine to L-α-lysophosphatidylcholine and a fatty acid per min at pH 8.0 at 37 °C.
Stato fisico
Suspension in 3.2 M (NH4)2SO4 solution, pH 5.5
Risultati analitici
Protein determined by biuret.
Inibitore
N° Catalogo
Descrizione
Determinazione del prezzo
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
B van den Berg et al.
The EMBO journal, 14(17), 4123-4131 (1995-09-01)
The lipolytic enzyme phospholipase A2 (PLA2) is involved in the degradation of high-molecular weight phospholipid aggregates in vivo. The enzyme has very high catalytic activities on aggregated substrates compared with monomeric substrates, a phenomenon called interfacial activation. Crystal structures of
Knut Kölbel et al.
Biophysical chemistry, 206, 12-21 (2015-06-29)
Porcine pancreatic phospholipase A2, a small and disulfide rich protein, is extremely resistant against chemically or thermally induced unfolding. Despite this marked resistance, the protein displays broad unfolding transitions resulting in comparatively low apparent thermodynamic stability. Broad unfolding transitions may
R A Zager et al.
Proceedings of the National Academy of Sciences of the United States of America, 90(17), 8297-8301 (1993-09-01)
During hypoxic or ischemic renal tubular injury, phospholipase A2 (PLA2) induces membrane deacylation, causing fatty acid accumulation and phospholipid breakdown. Because these changes can compromise cellular integrity, PLA2 activity has been widely proposed as a critical mediator of hypoxic renal
Elena Venuti et al.
Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society, 16(6), 763-770 (2017-07-26)
Bile salt stimulated lipase (BSSL; Enzyme Commission (EC) number 3.1.1.13) has been a candidate triglyceridase for improving enzyme therapy for pancreatic insufficiency; however, its efficacy is near absent. We hypothesise that similarly to pancreatic lipase, BSSL is inhibited by phospholipids
B van den Berg et al.
Journal of biomolecular NMR, 5(2), 110-121 (1995-02-01)
The three-dimensional structure of porcine pancreatic PLA2 (PLA2), present in a 40 kDa ternary complex with micelles and a competitive inhibitor, has been determined using multidimensional heteronuclear NMR spectroscopy. The structure of the protein (124 residues) is based on 1854
Articoli
Instructions for working with enzymes supplied as ammonium sulfate suspensions
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.