F3055
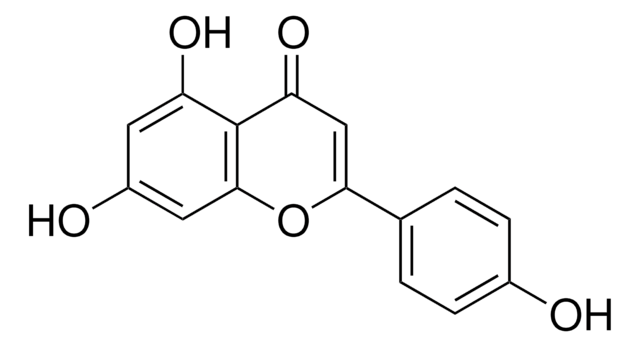
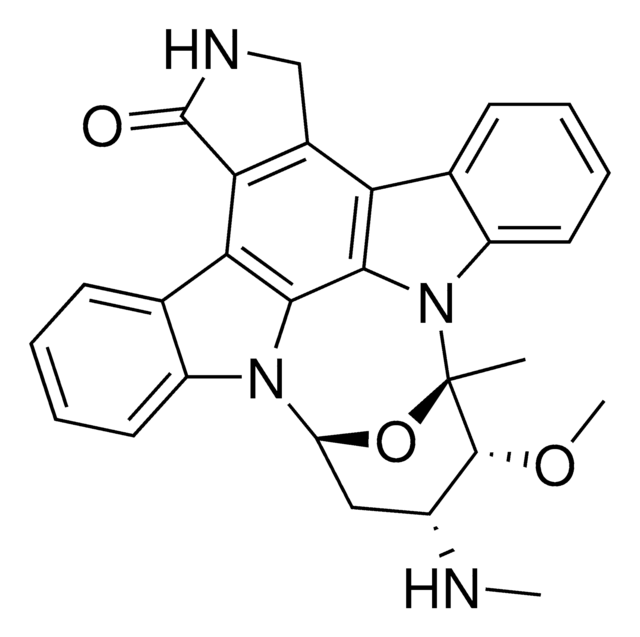
Flavopiridol
≥98% (HPLC), powder
Sinonimo/i:
(−)-2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3s,4r)-3-hydroxy-1-methyl-4-piperidinyl]-4h-1-benzopyran-4-one, L-86-8276, NSC-649890
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥98% (HPLC)
Stato
powder
Condizioni di stoccaggio
desiccated
Colore
white to light brown
Solubilità
H2O: ~2 mg/mL
DMSO: >5 mg/mL
Temperatura di conservazione
2-8°C
Stringa SMILE
OC1=C(C(C=C(C2=C(Cl)C=CC=C2)O3)=O)C3=C([C@H]4CCN(C)C[C@H]4O)C(O)=C1.Cl
InChI
1S/C21H20ClNO5.ClH/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22;/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3;1H/t12-,17+;/m0./s1
LGMSNQNWOCSPIK-LWHGMNCYSA-N
Informazioni sul gene
human ... CDK1(983) , CDK2(1017) , CDK4(1019) , CDK6(1021) , CDK7(1022) , CDK9(1025)
Applicazioni
- as a cyclin-dependent kinase 9 (CDK9) inhibitor to study its effects on histone H3 methylation at lysine 36 (H3K36) and deactivation of transcription in porcine fetal fibroblasts
- as an RNA polymerase inhibitor to study its effects on hepatic cells
- as RNA transcription inhibitor to study its effects on euchromatin coarsening in zebrafish embryo
Azioni biochim/fisiol
Caratteristiche e vantaggi
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Human epithelial intestinal colonic organoids can be used as an alternative to Caco-2 drug permeability assays for drug screening and compound toxicity testing.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.